Zinc: Valence Electrons And Their Role In Chemistry
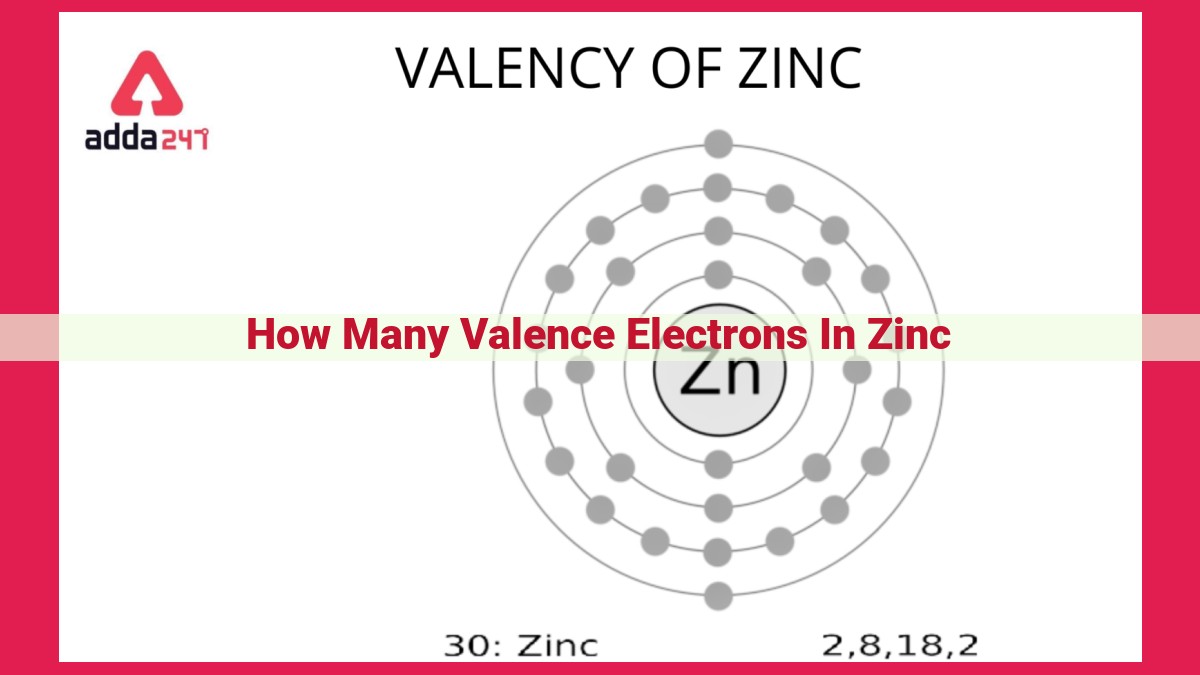
Zinc, with an atomic number of 30, has an electronic configuration of [Ar] 3d10 4s2. This indicates that zinc possesses two valence electrons in its outermost 4s energy level. Valence electrons play a crucial role in chemical bonding and reactivity, influencing zinc’s ability to form compounds and interact with other elements. Understanding the number of valence electrons in zinc is essential for studying its chemical properties and biological functions.
Zinc’s Atomic Number: A Cornerstone of Its Properties
Every element in the vast tapestry of our universe is defined by its unique atomic number, a fundamental characteristic that holds the key to understanding its chemical and physical properties. Zinc, an essential mineral for life as we know it, is no exception. Its atomic number, 30, tells a captivating tale that sets the stage for its remarkable versatility.
Zinc’s atomic number reveals that it possesses 30 protons within its tiny nucleus. These protons carry a positive electrical charge, which is balanced by an equal number of electrons that orbit the nucleus. The number of protons and electrons in an atom determines its elemental identity.
Atomic structure is a fascinating concept that governs the arrangement of these subatomic particles. Protons reside in the nucleus, while electrons occupy distinct energy levels or orbitals that surround the nucleus. The distribution of electrons within these orbitals determines an element’s chemical behavior.
Zinc’s atomic structure plays a pivotal role in its chemical reactivity. Its valence electrons, located in the outermost energy level, are the key players when it comes to forming chemical bonds with other atoms. These valence electrons determine the element’s chemical reactivity and explain why zinc exhibits a wide range of oxidation states in various compounds.
By unraveling the secrets of zinc’s atomic number, we gain invaluable insights into the fundamental properties that make this element so versatile and essential. Its atomic structure serves as the foundation for its chemical interactions, paving the way for its multifaceted roles in biological systems and industrial applications.
Zinc’s Electronic Configuration: Unveiling the Building Blocks of the Metal
In the world of chemistry, understanding the electronic structure of elements is crucial for unraveling their properties and behavior. Among these elements, zinc stands out as a versatile metal with significant biological and industrial applications. To fully grasp zinc’s fascinating chemistry, we embark on a journey to explore its electronic configuration.
Imagine zinc atoms as miniature solar systems, with a nucleus at their core, surrounded by orbiting electrons. The number of protons within the nucleus defines the atomic number, which for zinc is 30. This signifies the presence of 30 positively charged protons and 30 negatively charged electrons.
Electrons occupy specific energy levels, known as orbitals, which are arranged in shells around the nucleus. The outermost shell, the valence shell, plays a pivotal role in chemical bonding and reactivity. For zinc, the electronic configuration is denoted as [Ar] 3d10 4s2.
Let’s decipher this notation:
-
[Ar] represents the electron configuration of argon, the noble gas preceding zinc in the periodic table. This indicates that zinc’s inner shells are the same as argon’s, with 18 electrons comfortably filling the 1s, 2s, 2p, and 3s orbitals.
-
3d10 denotes the 3d subshell, which contains 10 electrons. The superscript “10” signifies that all five 3d orbitals are fully occupied, each holding two electrons.
-
4s2 represents the 4s subshell, which holds 2 electrons. These two electrons reside in the outermost energy level and are crucial for chemical bonding.
In summary, zinc’s electronic configuration tells us that it has 10 filled d orbitals, ensuring stability, and 2 valence electrons in the 4s subshell. These valence electrons are the key players in zinc’s chemical interactions, influencing its reactivity and ability to form bonds with other elements. Understanding zinc’s electronic configuration lays the foundation for comprehending its diverse roles in biological systems and industrial applications.
Zinc’s Valence Electrons: Unlocking the Secrets of Chemical Bonding
Zinc, a fascinating metal with a silvery-blue sheen, plays a vital role in various biological processes. Valence electrons, the electrons in the outermost energy level of an atom, hold the key to understanding zinc’s chemical behavior and reactivity.
In the case of zinc, it boasts two valence electrons occupying the 4s energy level. These electrons are crucial for forming chemical bonds, the forces that hold atoms together to create molecules and compounds. The valence shell, which accommodates the valence electrons, determines an element’s chemical properties.
Valence electrons are like the social butterflies of the atomic world, eagerly interacting with other atoms to form stable configurations. They participate in chemical bonding by sharing, transferring, or accepting electrons to achieve a balanced electronic arrangement. This dance of valence electrons leads to the formation of countless molecules that shape our world, from water to DNA.
Understanding valence electrons is essential for unraveling the mysteries of zinc’s chemical properties and its role in biological systems. It explains why zinc forms particular compounds, how it reacts with other elements, and its involvement in enzymatic reactions that sustain life. By delving into the world of valence electrons, we unlock the secrets of this versatile metal and its profound influence on our lives.