Structure And Properties Of Water: The Role Of Hydrogen And Oxygen
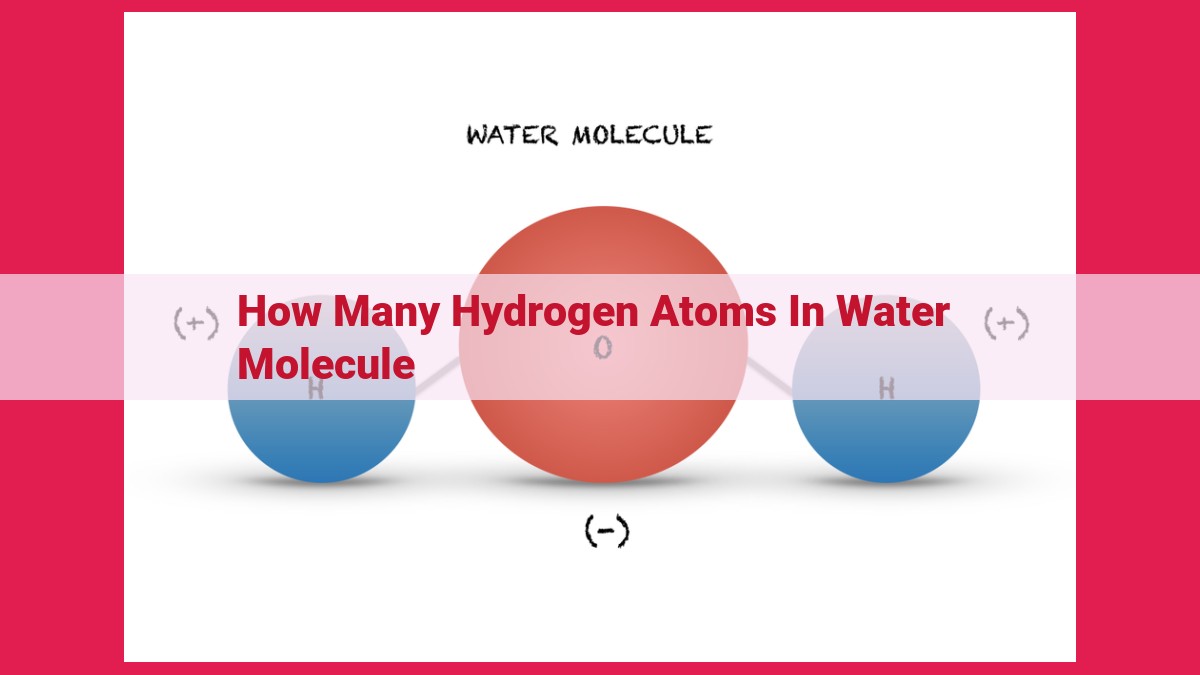
A water molecule (H2O) comprises two hydrogen atoms and one oxygen atom. Hydrogen, with an atomic number of 1, contributes one proton and one electron to the covalent bond formed with oxygen. In the water molecule, the hydrogen atoms share electrons with the oxygen atom, resulting in a polar structure. This polarity, along with hydrogen bonding, imparts unique properties to water, influencing its behavior and interactions in various chemical and biological processes.
Understanding the Essence of Water: Unveiling the Number of Hydrogen Atoms
In the realm of science, deciphering the composition of water molecules holds profound significance. After all, water sustains life on Earth, shapes ecosystems, and plays a pivotal role in countless chemical and biological processes. This blog post embarks on a captivating journey to unravel the enigma surrounding a fundamental aspect of water’s composition: the number of hydrogen atoms it harbors.
As we delve into this exploration, we’ll not only unravel this specific question but also gain a deeper appreciation for the intricate web of atoms and molecules that orchestrate the very essence of water.
Understanding Hydrogen
- A. Atomic Number and Proton
- Define atomic number and proton.
- Explain that hydrogen has an atomic number of 1 and contains one proton.
- B. Subatomic Particles and Structure
- Describe the subatomic particles of hydrogen: protons, electrons, and neutrons.
- Explain the structure of a hydrogen atom, including its nucleus and electrons.
Understanding Hydrogen: The Building Block of Water
In the captivating realm of chemistry, hydrogen emerges as an enigmatic element, holding the key to unraveling the secrets of water. This elusive gas, the lightest and most abundant in the universe, plays a crucial role in shaping the very essence of our planet.
Atomically, hydrogen embodies simplicity. Its atomic number of 1 indicates that it contains a single proton within its nucleus, the very heart of the atom. This solitary proton defines hydrogen’s fundamental identity and distinguishes it from all other elements.
Beneath the proton’s embrace lies a cloud of electrons, the subatomic particles that dance around the nucleus. Hydrogen atoms, with their solitary electron, prefer to pair up with other atoms to achieve stability. This electron-sharing behavior, known as covalent bonding, is the driving force behind hydrogen’s ability to form molecules, including the most ubiquitous one on Earth: water.
Water Molecule Formation: A Dance of Covalent Bonds and Hydrogen Bonding
In the realm of chemistry, understanding the composition of water molecules holds immense significance. Water, the elixir of life, is composed of two hydrogen atoms and one oxygen atom, forming a molecule represented as H2O. To unravel the mysteries of this essential molecule, let’s delve into the intricate dance of covalent bonds and hydrogen bonding.
Covalent Bond: The Union of Atoms
Covalent bonds are partnerships between atoms, formed when they share electrons. In the case of water, hydrogen atoms, each with one electron, join forces with an oxygen atom, which has six electrons. The hydrogen atoms contribute one electron each, while the oxygen atom shares two of its electrons. This electron-sharing marathon results in a stable embrace, forming a water molecule.
Dipolarity and H-Bonding: A Network of Attractions
The water molecule, though seemingly simple, exhibits a remarkable dipole. Due to the unequal sharing of electrons between hydrogen and oxygen, the molecule acquires a partial positive charge on the hydrogen side and a partial negative charge on the oxygen side. This polarity endows water with unique properties.
Hydrogen bonding emerges as a crucial force between water molecules. The positive hydrogen atoms of one molecule are irresistibly drawn to the negative oxygen atoms of another, forming a network of intermolecular attractions. These hydrogen bonds are the silent architects behind water’s high boiling point, cohesion, and adhesion.
In essence, two hydrogen atoms, bound to an oxygen atom through covalent bonds, give rise to a water molecule. This molecule, with its inherent dipole and propensity for hydrogen bonding, plays a vital role in shaping the properties and behavior of water. From its life-giving qualities to its industrial applications, water’s remarkable versatility stems from the intricate dance of its constituent atoms.
Role of Hydrogen in Water Molecule Formation
In the realm of chemistry, understanding the composition of substances is paramount, especially when it comes to a life-sustaining molecule like water. At the heart of a water molecule lies hydrogen, playing a crucial role in its formation.
A Chemical Reaction: The Birth of a Water Molecule
The formation of a water molecule is a captivating chemical dance between two hydrogen atoms and one oxygen atom. This intricate process is governed by the fundamental principles of covalent bonding. Covalent bonds arise when atoms share electrons, creating a strong attraction that binds them together.
Hydrogen’s Contribution: Sharing is Bonding
In the case of water, hydrogen atoms generously share their single electron with an oxygen atom that craves two electrons. This electron-sharing arrangement forms the covalent bonds that hold the water molecule together. The result is a highly stable configuration where all atoms have satisfied their electronic cravings.
This electron-sharing process, however, creates an interesting twist in the water molecule’s structure. The oxygen atom, with its greater electronegativity, exerts a stronger pull on the shared electrons. This imbues the oxygen end of the molecule with a slight negative charge, while the hydrogen ends acquire a slight positive charge.
Hydrogen’s Role: Beyond Bonding
The polarity of the water molecule, arising from the unequal sharing of electrons, has profound implications for its behavior. It’s this polarity that enables water molecules to form hydrogen bonds with their neighbors. Hydrogen bonding is a special type of attraction that occurs between the positive hydrogen end of one molecule and the negative oxygen end of another molecule.
These hydrogen bonds create a network of interconnected water molecules, giving water its unique properties. This hydrogen-bonded network is responsible for water’s high surface tension, heat capacity, and ability to dissolve a wide range of substances.
In essence, hydrogen atoms not only form the backbone of water molecules but also play a pivotal role in determining their unique characteristics. Their involvement in covalent bonding and hydrogen bonding makes water an indispensable component of life on our planet.