Unveiling The Water Molecule’s Unique Shape: A Guide To Its Essential Properties
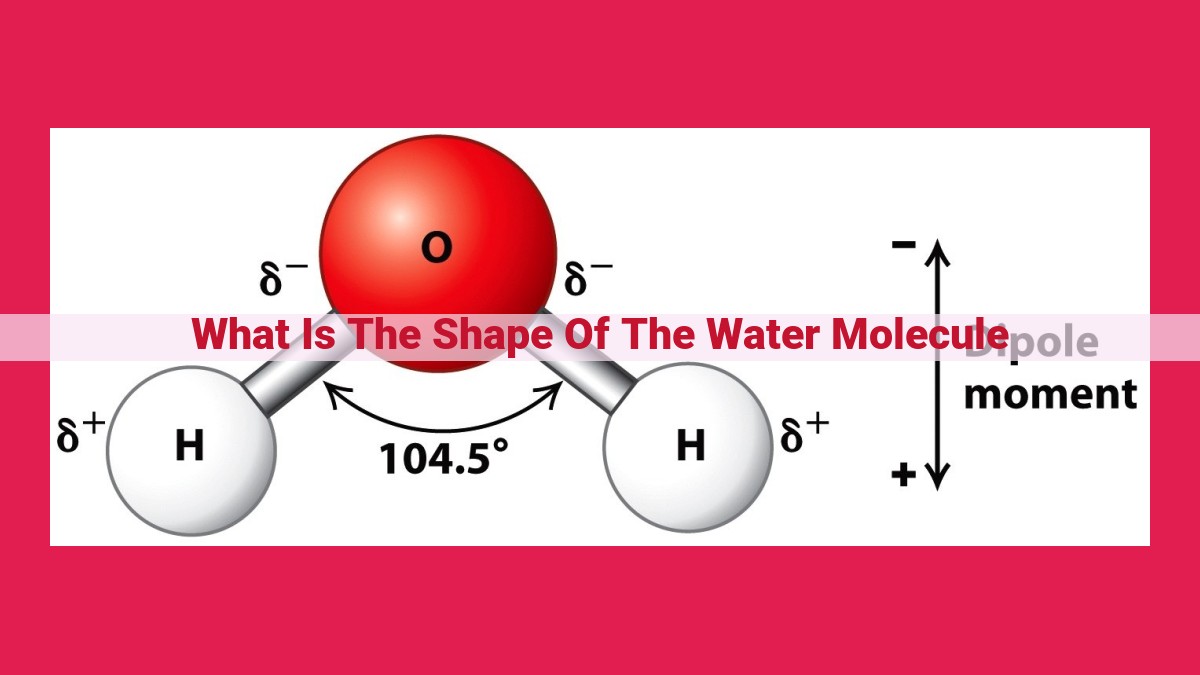
The water molecule’s unique bent shape is determined by the repulsion between its electron pairs. Using VSEPR theory, the four electron pairs (two bonding pairs and two lone pairs) around the central oxygen atom arrange themselves to minimize this repulsion. The lone pairs exert a stronger repulsive force than the bonding pairs, pushing the bonding pairs closer together and resulting in a bent molecular geometry. This shape, with a bond angle of approximately 104.5 degrees, is crucial for the water molecule’s polarity, hydrogen bonding capabilities, and other properties that make it essential for life on Earth.
The Shape of the Water Molecule: Unraveling the Mysteries of H2O
Embarking on a journey to understand the molecular shape of water opens up a fascinating world of chemistry. The shape of molecules plays a crucial role in determining their properties and behavior, and water is no exception. This seemingly simple molecule holds the key to many of life’s essential processes.
To unravel the mysteries of water’s shape, we delve into the realm of molecular geometry. Valence shell electron pair repulsion (VSEPR) theory guides us in understanding how electrons arrange themselves around atoms, dictating the geometry of molecules.
In the case of water (H2O), VSEPR theory reveals that the oxygen atom at the heart of the molecule possesses four electron pairs: two involved in bonding with hydrogen atoms and two lone pairs. The repulsive forces between these electron pairs shape the geometry of the water molecule, resulting in its signature bent or V-shaped structure.
This unique shape has profound implications. The separation of charges within the water molecule, due to the unequal distribution of electrons, creates a dipole moment. This polarity enables water molecules to interact with each other through hydrogen bonds, forming a vast network of intermolecular connections.
VSEPR Theory and Electron Pair Geometry: Unraveling the Shape of the Water Molecule
In the realm of chemistry, molecular shape plays a pivotal role in determining a molecule’s properties and behavior. The water molecule, a seemingly simple compound, holds a fascinating tale of its unique bent shape, unraveling the intricacies of molecular geometry and electron pair interactions.
Enter VSEPR theory (Valence Shell Electron Pair Repulsion), a fundamental tool in predicting the shape of molecules. This theory posits that the electrons surrounding an atom will arrange themselves to minimize electrostatic repulsion. In the case of the water molecule, the central oxygen atom has four electron pairs surrounding it, two of which are lone pairs and the remaining two involved in covalent bonds with hydrogen atoms.
These electron pairs are arranged in a tetrahedral electron pair geometry, forming a virtual tetrahedron with the oxygen atom at its center. However, the presence of the lone pairs introduces a twist in the tale. Lone pairs, due to their negative charge, exert a stronger repulsive force compared to bonding pairs. As a result, the tetrahedron is distorted, leading to the bent shape characteristic of the water molecule.
The lone pairs occupy a larger space than the bonding pairs, pushing the hydrogen atoms closer together. This arrangement creates a molecular structure where the hydrogen-oxygen-hydrogen bond angle is approximately 104.5 degrees, significantly deviating from the 90 degrees predicted by tetrahedral geometry.
It is this unique bent shape that endows the water molecule with its polarity, a separation of charges. The oxygen atom, with its concentrated electron density, exhibits a partial negative charge, while the hydrogen atoms carry a partial positive charge. This polarity enables the water molecule to form hydrogen bonds with other molecules, leading to its remarkable solvent properties and its essential role in various biological processes.
Lone Pairs and the Bent Shape of Water
In the fascinating tapestry of chemistry, understanding molecular shape plays a pivotal role in deciphering the properties and behaviors of molecules. Among the myriad of molecules that captivate scientists’ attention, water (H₂O) stands out as a quintessential example. Its unique bent shape is a captivating tale of electronic dance, where the repulsive forces of lone pairs mold its geometric destiny.
Electron Pair Geometry: A Prelude to Shape
The shape of a molecule is intimately linked to its electron pair geometry, which describes the spatial arrangement of electron pairs surrounding its central atom. In the case of water, oxygen proudly holds the central stage, with four electron pairs gravitating around it. Two of these pairs are covalently bonded to hydrogen atoms, forming the covalent bonds that hold the molecule together. The remaining two electron pairs refuse to participate in bonding, preferring to remain as lone pairs, their negative charges repelling one another.
Lone Pairs: The Shape-Shifters
The presence of these lone pairs exerts a profound influence on the molecular shape of water. Their mutual repulsion forces the bonded electron pairs to move away from them, creating a bent shape. Imagine a see-saw with the central oxygen atom as the fulcrum. The bonded hydrogen atoms, like children on opposite ends, balance each other out. However, the two lone pairs, perched on one side, disrupt this equilibrium, causing the seesaw to tilt, giving the water molecule its characteristic V-shaped geometry.
The Bent Shape: A Consequence of Electronic Dance
Thus, the bent shape of water emerges as a consequence of the electronic dance between the lone pairs and the bonded electron pairs. This shape is not merely an aesthetic curiosity; it has profound implications for water’s physical and chemical properties, making it a key player in the symphony of life. From its high surface tension to its ability to dissolve a vast array of substances, the bent shape of water underpins its role as the elixir of life.
Hydrogen Bonds and Molecular Polarity: The Water Molecule’s Quirky Secret
The water molecule, an unassuming compound composed of two hydrogen atoms and one oxygen atom, conceals a remarkable secret within its deceptively simple structure: the power of hydrogen bonding. This unique ability to form hydrogen bonds stems from the molecule’s polarity.
Dipole Moment and Separation of Charges
The water molecule’s polarity arises from the unequal distribution of electrical charge within its atoms. Oxygen, known for its affinity for electrons, exerts a stronger pull on the electrons shared with hydrogen than hydrogen does. This creates a separation of charges, where the oxygen atom becomes slightly negative and the hydrogen atoms acquire a slight positive charge.
Formation of Hydrogen Bonds: Dipole Attractions
The polarized nature of the water molecule gives rise to dipole interactions. When the negative end (the oxygen atom) of one water molecule approaches the positive end (the hydrogen atom) of another, an electrostatic attraction occurs. This interaction forms a bond known as a hydrogen bond.
Hydrogen bonds are crucial to the unique properties of water. They enable water molecules to cluster together, creating a cohesive network. This, in turn, contributes to water’s high surface tension, making it difficult to break its surface.
Impact on Water’s Properties
The hydrogen bonding ability of water profoundly influences its physical and chemical properties. It explains water’s high boiling point (100°C), as it takes considerable energy to break the hydrogen bonds holding water molecules together. Additionally, water’s excellent solvent capabilities arise from its ability to form hydrogen bonds with dissolved substances.
The bent shape of the water molecule, combined with its polarity and hydrogen bonding ability, endows it with a remarkable set of properties. These properties not only make water essential for life on Earth but also contribute to its diverse applications in chemistry and industry. Understanding the shape and polarity of the water molecule provides a deeper appreciation for its multifaceted nature and its indispensable role in our world.
Oxygen Atom Hybridization: Unveiling the Hidden Geometry of Water
In our exploration of the intriguing shape of the water molecule, we venture into the realm of quantum mechanics to understand the role of oxygen atom hybridization. Hybridization, a fundamental concept in chemistry, describes the blending of atomic orbitals to form new hybrid orbitals with distinct shapes and energies.
For the oxygen atom in the water molecule, sp³ hybridization occurs. In this process, one 2s and three 2p orbitals combine to form four sp³ hybrid orbitals. These hybrid orbitals arrange themselves in a tetrahedral geometry, with the bonding pairs of electrons occupying four corners of a tetrahedron.
However, the presence of two lone pairs of electrons on the oxygen atom disrupts this ideal tetrahedral shape. Lone pairs, which are electron pairs not involved in bonding, occupy more space than bonding pairs and exert a repulsive force on them. As a result, the tetrahedral geometry becomes distorted, leading to the molecule’s bent shape.
The lone pairs push against the bonding pairs, causing them to move closer to each other. This results in the formation of two bond angles of approximately 104.5 degrees, rather than the 109.5 degrees expected for a perfect tetrahedron. The oxygen atom’s electron configuration, with its two lone pairs and sp³ hybridization, thus determines the characteristic bent shape of the water molecule.