Water Molecule: Composition, Bonding, And Structure For Seo
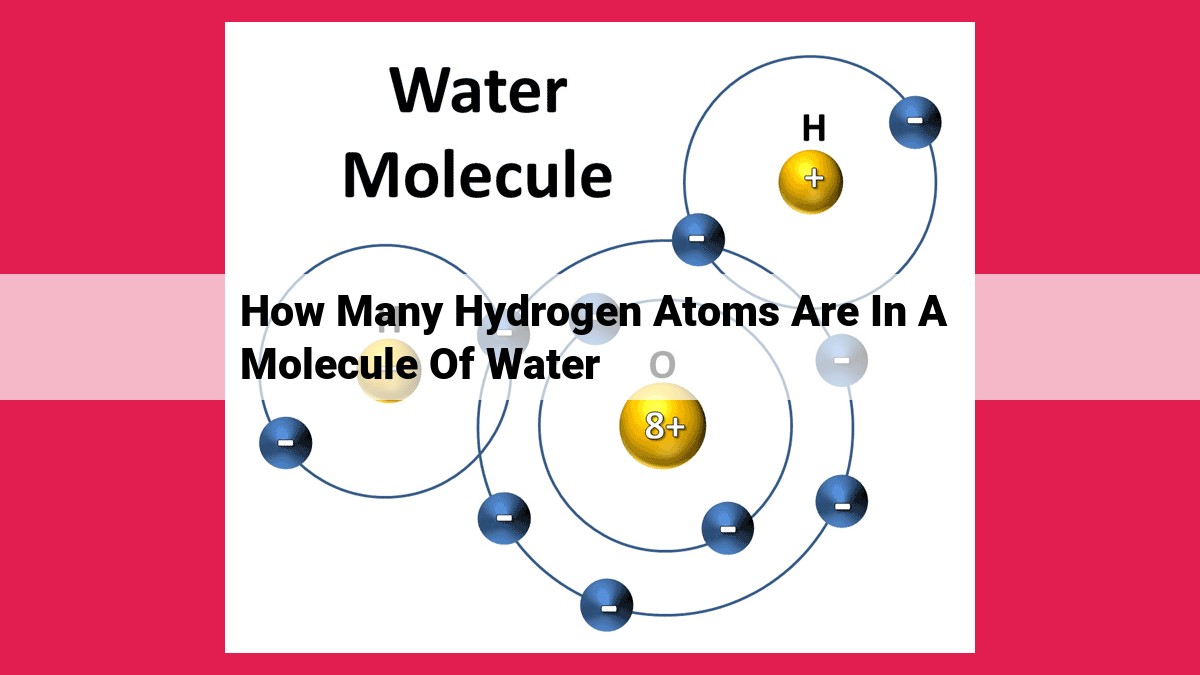
A water molecule consists of two hydrogen atoms and one oxygen atom. This is expressed by the chemical formula H2O. The number of hydrogen atoms can be deduced from the stoichiometry of water, where there are two hydrogen atoms for every one oxygen atom. The hydrogen atoms are covalently bonded to the oxygen atom, forming a molecular structure with a bent shape.
Understanding the Hydrogen Atom: A Journey into the Heart of Matter
In the vast tapestry of elements that weave the fabric of our universe, hydrogen, the simplest and most ubiquitous of them all, holds a profound significance. It is the foundational building block of the cosmos, accounting for an astounding 75% of all matter. To unravel the mysteries of the universe, we must first understand the fundamental nature of the hydrogen atom: a dance of subatomic particles.
At the heart of every hydrogen atom lies the nucleus, a dense, positively charged center. Within this nucleus resides a single proton, a massive particle carrying a positive charge. Like a miniature sun, the nucleus exerts an immense gravitational pull that binds a lone electron to its orbit. This electron, a weightless particle with a negative charge, is the constant companion of the proton, whirling around it at incredible speeds.
The interaction between these two fundamental particles defines the chemical bonding that gives rise to all the diverse substances in our world. The electron’s intrinsic desire to occupy a lower energy level drives it towards the nucleus. However, the electrostatic repulsion between the negatively charged electron and the positively charged proton counterbalances this attraction, maintaining a delicate equilibrium within the atom.
Exploring the Water Molecule: A Deeper Dive into Life’s Elixir
At the heart of life lies a remarkable molecule: water. Its humble yet pivotal structure holds secrets that sustain the tapestry of life on Earth. Let’s unveil the fascinating architecture of this ubiquitous substance.
The water molecule is a trio of interconnected atoms: two hydrogen atoms and a central oxygen atom. The oxygen atom, like a cosmic magnet, draws the hydrogen atoms close, forming a tetrahedral arrangement. However, due to the oxygen atom’s inherent electronegativity, it exerts a stronger pull, creating a polar covalent bond between the atoms.
Picture this: the oxygen atom, with its six electrons, becomes slightly negative, while the two hydrogen atoms, each with a single electron, become slightly positive. This polarity, akin to a magnetic dance, is crucial for water’s unique properties.
The dance between the water molecule’s atoms creates hydrogen bonds, which are attractive forces between the partial positive charge of a hydrogen atom and the negative charge near the oxygen atom. These bonds, like invisible threads, form a network, linking water molecules and giving them their cohesive nature.
The polarity and hydrogen bonding of water molecules play a pivotal role in its solvating properties. Water readily dissolves ionic compounds, separating positively and negatively charged ions. It also forms hydration shells around molecules, enabling their transport and chemical reactions in biological systems.
In summary, the water molecule is a masterpiece of molecular architecture, where two hydrogen atoms and an oxygen atom dance in a symphony of polarity and hydrogen bonding. This dance gives water its unique properties, making it an essential component of life’s vibrant tapestry.
Decoding the Chemical Formula of Water
Every glass of water we drink, every drop that sustains life on Earth, is composed of a simple yet fascinating molecule: H2O. This three-letter formula holds the secret to understanding the fundamental building block of our very existence.
The Significance of H2O
The chemical formula H2O is like a molecular fingerprint, uniquely identifying water as a distinct substance. It indicates that each water molecule consists of two hydrogen atoms and one oxygen atom. This precise arrangement of atoms determines the unique properties of water, making it essential for life.
Understanding the Formula
The number in front of each atomic symbol represents the number of atoms in the molecule. So, H2 denotes two hydrogen atoms, and O represents one oxygen atom. This formula indicates that in every molecule of water, there are exactly two hydrogen atoms for every one oxygen atom.
Molecular Formula as Chemical Composition
The chemical formula of a molecule serves as a roadmap for its chemical composition. It provides a precise blueprint, showing the exact number and type of atoms present. H2O, therefore, tells us that water is composed of hydrogen and oxygen atoms and that it contains two hydrogen atoms per oxygen atom.
In conclusion, the chemical formula H2O is not just a sequence of letters and numbers. It is a key to unlocking the secrets of water, revealing its molecular structure and chemical composition. This understanding empowers us to appreciate the importance of water and its role in sustaining life on Earth.
Determining the Number of Hydrogen Atoms in Water
- State that a water molecule contains two hydrogen atoms.
- Explain the stoichiometry of water and the 2:1 ratio of hydrogen atoms to oxygen atoms.
- Discuss the use of chemical symbols “H” and “O” to represent hydrogen and oxygen atoms, respectively.
Unveiling the Secrets of Water: Delving into a Water Molecule
Our journey into the realm of chemistry begins with the fundamental building block of the universe: the atom. Let’s focus our lens on the simplest and most abundant atom –hydrogen. This tiny particle, with its positively charged proton and negatively charged electron orbiting around it, plays a pivotal role in the formation of molecules.
Now, let’s shift our gaze to a molecule that’s essential for life on Earth: water. This unassuming molecule, composed of two hydrogen atoms and a single oxygen atom, is the lifeblood of our planet.
The chemical formula of water, H2O, provides a blueprint for its molecular makeup. The “H” represents hydrogen, and the “O” signifies oxygen. This formula communicates that, in a water molecule, two hydrogen atoms unite with one oxygen atom.
The stoichiometry of water, the precise ratio of its constituent atoms, is crucial to its properties. For every one oxygen atom, there are two hydrogen atoms. This 2:1 ratio is the cornerstone of water’s chemical identity.
In everyday language, we often refer to water as “H2O.” This shorthand notation serves as a reminder of the exact number of hydrogen atoms present in each water molecule. It underscores the fundamental principle that the number of atoms of each element in a compound is fixed and invariable.
Delving into the structure of a water molecule, we discover that the oxygen atom occupies the central position. The hydrogen atoms are positioned on either side of the oxygen atom, forming covalent bonds with it. These bonds are a result of the sharing of electrons between the hydrogen and oxygen atoms, creating a stable molecular structure.
Understanding the precise number of hydrogen atoms in a water molecule is not just a matter of academic curiosity. It’s a fundamental concept that underpins our understanding of water’s properties and its role in countless chemical reactions.
From the depths of the ocean to the heights of the sky, water permeates our existence. Its molecular structure, with its two hydrogen atoms and one oxygen atom, is a testament to the intricate tapestry of the natural world. By unraveling the secrets of water, we gain a deeper appreciation for the building blocks that make up our world.