Visualize Molecular Structures And Conformational Analysis With Vsepr Theory
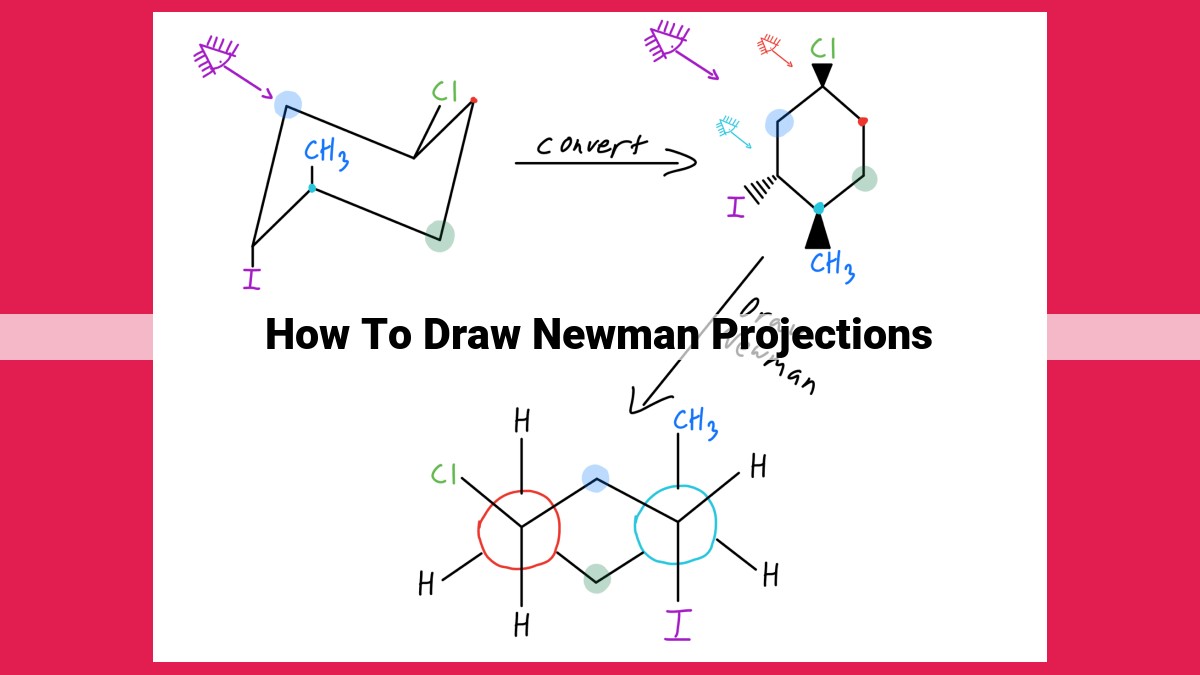
- Determine the molecular geometry using VSEPR theory. 2. Choose a bond of interest and align it vertically in a circle. 3. Represent atoms and groups as circles and wedges, respectively. 4. Rotate the molecule around the bond of interest to view different conformations. 5. Draw lines or wedges to represent bonds and atoms behind the front carbon, keeping their correct spatial orientation and relationships.
Molecular Geometry: Unveiling the 3D Landscape of Molecules
In the realm of chemistry, understanding the three-dimensional arrangement of atoms within molecules is crucial. This arrangement, known as molecular geometry, influences a molecule’s reactivity, physical properties, and biological function. Three key concepts underpin molecular geometry: bond angles, bond lengths, and hybridization.
Bond Angles:
The bond angle is the angle formed between any two adjacent bonds sharing a common atom. It determines the shape of the molecule. For example, in water (H2O), each hydrogen atom forms a bond angle of 104.5 degrees with the oxygen atom, giving the molecule a bent shape.
Bond Lengths:
The bond length is the distance between the nuclei of two bonded atoms. It reflects the strength and nature of the chemical bond. The bond length between two carbon atoms in a double bond is typically shorter than that in a single bond due to the presence of a stronger double bond.
Hybridization:
Hybridization is a fundamental concept that describes the mixing of atomic orbitals to form new hybrid orbitals. These hybrid orbitals have specific shapes and orientations that influence both bond angles and bond lengths. For instance, the hybridization of carbon atoms in methane (CH4) results in four equivalent sp3 hybrid orbitals that form four strong and equally spaced covalent bonds, giving the molecule its tetrahedral shape.
Understanding molecular geometry provides a solid foundation for comprehending the behavior and properties of molecules. It enables chemists to predict the shape, reactivity, and even biological activity of molecules, guiding drug design, materials science, and many other fields.
Unveiling Molecular Structure: A Journey through Lewis Structures, Resonance, and Molecular Orbitals
In the realm of chemistry, understanding the structure of molecules is paramount to deciphering their properties and behavior. Among the many tools that have been developed to describe molecular structures, Lewis structures stand out as a fundamental and widely used framework.
Named after the renowned chemist Gilbert Newton Lewis, Lewis structures are a visual representation of the arrangement of electrons within a molecule. They depict the shared and unshared** electron pairs that form chemical bonds between atoms. By scrutinizing the Lewis structure of a molecule, we can gain insights into its *geometry, polarity, and stability.
Another important concept in molecular structure is that of resonance structures. Certain molecules can exist in multiple equivalent electronic configurations, each represented by a different Lewis structure. These resonance structures contribute to the overall electronic structure of the molecule, providing a more complete description of its bonding and properties.
The concept of molecular orbitals takes us a step further, delving into the quantum mechanical nature of electrons. Molecular orbitals describe the regions around a molecule where the electrons are most likely to be found. These regions are defined by mathematical functions and provide a more detailed picture of the electron distribution within a molecule, enabling us to better understand its chemical behavior.
Finally, the Valence Shell Electron Pair Repulsion (VSEPR) theory helps us predict the three-dimensional geometry of molecules based on the arrangement of their electron pairs. VSEPR theory considers the repulsive forces between electron pairs, assuming that the molecule will adopt a geometry that minimizes these repulsions. By considering the number and arrangement of electron pairs, we can infer the bond angles and molecular shape of countless compounds.
By unraveling the intricacies of Lewis structures, resonance structures, molecular orbitals, and VSEPR theory, we uncover the hidden order within the fascinating world of molecules. These concepts serve as indispensable tools for chemists, providing a deeper understanding of the structure, properties, and behavior of the chemical compounds that shape our universe.
Newman Projection: Visualizing Molecular Conformations
Understanding the three-dimensional arrangement of atoms within a molecule is critical in chemistry. One powerful tool that helps us visualize this is the Newman projection.
Conformational Analysis
Molecules exist in different shapes or conformations due to the free rotation around single bonds. Conformational analysis examines these conformations and their energy differences. The lowest energy conformation is typically the most stable.
Dihedral Angles
To describe the orientation of one carbon-carbon bond relative to another, we use dihedral angles. These angles are measured in degrees from 0° to 360°. When two adjacent carbon atoms are eclipsed (directly facing each other), the dihedral angle is 0°. When they are staggered (offset by 60°), the dihedral angle is 60°.
Purpose of Newman Projections
Newman projections are two-dimensional representations that allow us to visualize molecular conformations along a specific carbon-carbon bond. They help us determine the dihedral angles and predict the stability of different conformations.
Drawing Newman Projections: A Step-by-Step Guide
- Choose the Bond of Interest: Identify the carbon-carbon bond you want to focus on.
- Represent Atoms and Groups: Draw a dot for each atom at the bond of interest and lines for any groups attached to them.
- Rotate the Molecule: Rotate the molecule so that the bond of interest is vertical. The front atom is represented by a dot, while the back atom is hidden behind it.
- Determine Dihedral Angles: Look at the atoms or groups attached to the front and back atoms. Dihedral angles can be measured by rotating one atom 60° clockwise or counterclockwise.
- Represent Perspective: Using dashes (—) and wedges (/), indicate the atoms or groups that are closer to (front) or farther from (back) you.
By following these steps, you can effectively draw Newman projections and gain insights into the three-dimensional structures of molecules.
Line-Bond Representation: Simplifying Molecular Structures
When it comes to visualizing the arrangement of atoms and bonds in molecules, line-bond representations offer a convenient way to depict their molecular structures. These representations are like blueprints for molecules, using lines to represent bonds and circles or dots to represent atoms.
However, line-bond representations have their limitations. They provide a two-dimensional view of the molecule, which can be misleading for complex molecules with three-dimensional structures. For example, a line-bond representation of water (H2O) shows two hydrogen atoms connected to an oxygen atom, but it doesn’t show the tetrahedral shape of the molecule.
Despite their limitations, line-bond representations remain a valuable tool for visualizing molecular structures. They are simple to draw and can convey essential information about the connectivity and bonding of atoms within a molecule. This makes them particularly useful for quickly comparing the structures of different molecules or for representing large molecules where more detailed representations would be too complex.
Here’s a quick example to illustrate:
- Line-bond representation of methane (CH4):
H
|
H---C---H
|
H
This representation shows that methane has four hydrogen atoms bonded to a central carbon atom. However, it doesn’t provide any information about the three-dimensional arrangement of these atoms.
Wedge-Hash Representation: Unraveling Molecular Perspectives
In the realm of molecular structures, visualizing the three-dimensional arrangement of atoms is crucial for understanding their properties and behavior. Wedge-hash representation, a technique that employs wedges and dashes, offers a dynamic portrayal of molecular geometry.
Unlike line-bond representations that depict molecules on a flat surface, wedge-hash drawings provide a three-dimensional perspective. Wedges symbolize bonds protruding toward the viewer, while dashes indicate bonds receding away. This simple yet effective approach allows us to visualize the spatial relationships between atoms.
To create a wedge-hash representation, we begin by identifying the bond of interest. Then, the molecule is rotated so that the bond lies in the vertical plane. Atoms and groups attached to the front atom are represented by wedges, while those connected to the back atom are depicted by dashes.
By rotating the molecule and drawing multiple wedge-hash projections, we can visualize different conformations of the molecule. This technique is particularly valuable in understanding stereochemistry, the study of three-dimensional molecular arrangements.
In summary, wedge-hash representation provides a powerful tool to delve into the intricate world of molecular structures. By adding depth to molecular perspectives, we gain a deeper insight into their geometry, spatial relationships, and conformations.
Drawing Newman Projections: A Step-by-Step Guide for Visualizing Molecular Conformations
Comprehending the spatial arrangement of atoms within molecules is crucial in chemistry. Newman projections offer an intuitive way to visualize these three-dimensional structures, providing a clear understanding of molecular conformations.
Step 1: Choosing the Bond of Interest
Select the central bond that you want to focus on. This will serve as the axis of rotation for the Newman projection.
Step 2: Representing Atoms and Groups
Project the atoms or groups attached to the carbon atoms on either side of the central bond onto a horizontal line. The larger group is typically placed at the back.
Step 3: Rotating the Molecule
Rotate the molecule clockwise or counterclockwise along the central bond to obtain different perspectives. This allows you to visualize the relative positions of the groups around the central atom.
Additional Tips:
- Use circles to represent carbon atoms and letters to represent attached groups.
- Wedges indicate bonds projecting forward from the plane, while dashes indicate bonds projecting backward.
- Label the dihedral angles between the groups to quantify their relative orientation.
Example: Ethane
Let’s draw a Newman projection for ethane (CH3-CH3).
- Step 1: Central bond is C-C.
- Step 2: Project the CH3 groups onto the horizontal line, with the rear one larger.
- Step 3: Rotate the molecule to obtain the following Newman projection:
H H
| |
C---C
| |
H H
By following these steps, you can easily draw Newman projections to visualize and analyze the three-dimensional structures of molecules. This technique is essential for understanding molecular conformations, bond rotations, and stereochemistry.