Vanadium: Valence Electrons, Electron Configuration, And Chemical Properties
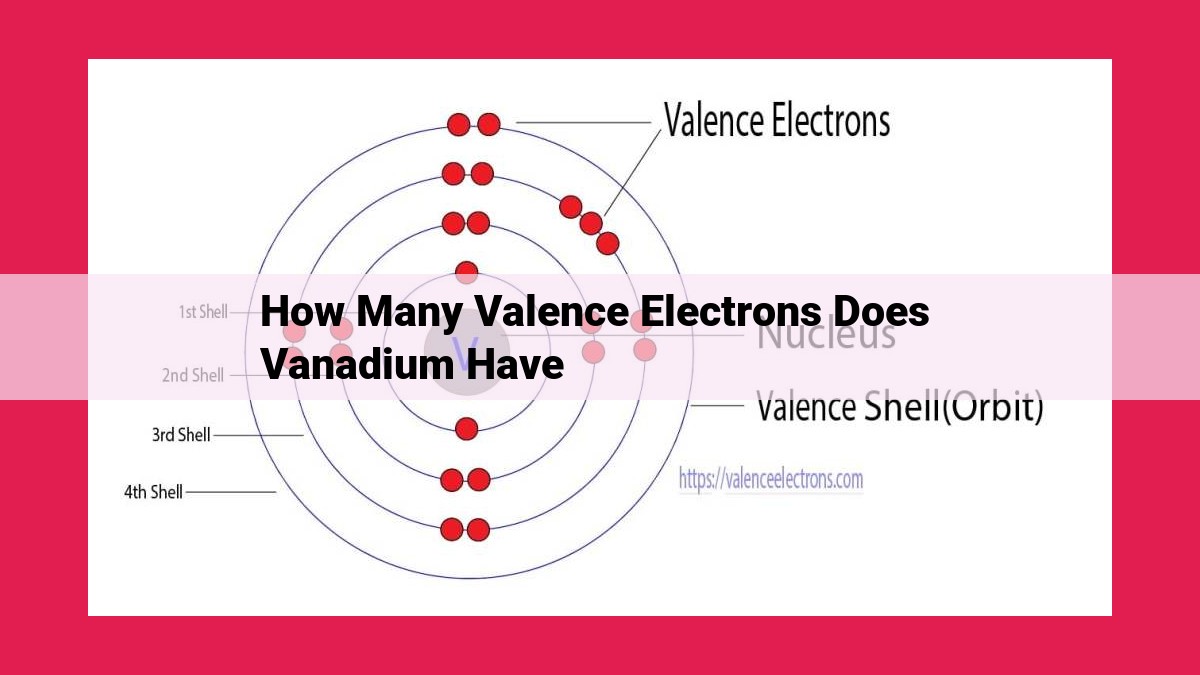
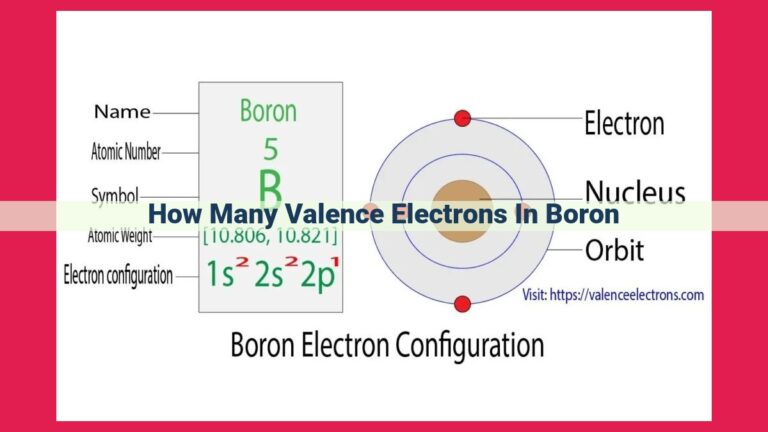
Vanadium, with an atomic number of 23, has 5 valence electrons. The electron configuration of vanadium is [Ar] 3d³ 4s², indicating 3 electrons in the 3d orbital and 2 in the 4s orbital. Valence electrons, located in the outermost energy level, play a crucial role in chemical bonding, determining the reactivity and behavior of vanadium in various chemical reactions.
Vanadium: Unveiling the Intriguing Metal with Five Valence Electrons
In the realm of metals, vanadium stands out with its unique properties and fascinating chemistry. Among its many attributes, the question of its valence electrons has intrigued scientists and enthusiasts alike. To delve into this captivating topic, let’s embark on a journey to comprehend the intricate world of vanadium’s electron configuration.
Defining Valence Electrons
Valence electrons, the gatekeepers of chemical bonding, reside in the outermost energy level of an atom. They play a pivotal role in determining the chemical reactivity and properties of an element. Understanding the number of valence electrons in vanadium is crucial for unlocking its potential in various applications.
Vanadium’s Atomic Identity: A Symphony of Protons and Electrons
Every atom is characterized by its unique atomic number, the defining trait that governs its elemental identity. For vanadium, this number is 23, signifying the presence of 23 protons in its nucleus. By the fundamental rules of electromagnetism, the number of electrons must balance the protons, leading us to the conclusion that vanadium also possesses 23 electrons.
Electron Configuration: Mapping the Atomic Landscape
The electron configuration of an element is a detailed blueprint that unveils the distribution of its electrons within specific atomic orbitals. Vanadium’s electron configuration is expressed as [Ar] 3d³ 4s², a cryptic yet insightful notation.
-
[Ar] represents the electron configuration of the noble gas argon, indicating that vanadium’s core electrons are arranged in a similar manner.
-
3d³ signifies the presence of three electrons in the 3d orbital, which belongs to the third energy level and possesses a distinctive dumbbell shape.
-
4s² indicates that two electrons reside in the 4s orbital, a spherical orbital in the fourth energy level.
Five Valence Electrons: The Key to Vanadium’s Versatility
Delving into the outermost energy level, we find vanadium’s five valence electrons. Three of these electrons occupy the 3d orbital, while the remaining two reside in the 4s orbital. These valence electrons are the architects of vanadium’s chemical reactivity, enabling it to form diverse bonds with various elements.
Vanadium, with its 23 protons and electrons, exhibits a unique electron configuration of [Ar] 3d³ 4s². This configuration bestows upon vanadium five valence electrons, the driving force behind its versatile chemical behavior. Understanding the valence electrons of vanadium is essential for harnessing its potential in various fields, ranging from alloys to energy storage systems.
Vanadium’s Atomic Number: Exploring the Heart of an Element
Have you ever wondered about the intricate world of elements? In this blog post, we embark on a captivating journey to unravel the secrets of vanadium, a fascinating metal. Specifically, we’ll delve into the question: How Many Valence Electrons Does Vanadium Have?
Atomic Number: The Key to Understanding
Every atom possesses a unique identity, defined by its atomic number. This number represents the number of protons, the positively charged particles, within its nucleus. Protons not only dictate an element’s chemical properties but also determine the number of electrons orbiting the nucleus.
Vanadium’s Atomic Number: Unveiling the Truth
Vanadium, our element of interest, boasts an atomic number of 23. This means that every vanadium atom has 23 protons in its nucleus. Concurrently, this also implies the presence of 23 electrons orbiting the nucleus.
The atomic number of vanadium stands as the cornerstone of its identity, influencing its chemical behavior and the number of electrons it possesses. With this knowledge, we can now delve deeper into the fascinating realm of electron configuration and uncover the secrets of vanadium’s valence electrons.
Unveiling the Secrets of Vanadium: A Journey to Its Electron Configuration
In the realm of chemistry, delving into the intricate world of elements is a captivating endeavor. Today, we embark on a mind-bending exploration of vanadium, an element often shrouded in mystery. Our quest: to unravel the secrets of its electron configuration.
The Atomic Number of Vanadium: A Gateway to Discovery
Every element in the universe possesses a unique atomic number, which serves as a key to understanding its inner workings. Vanadium, with an atomic number of 23, stands apart as a fascinating subject of our investigation. This crucial number tells us that vanadium’s atomic nucleus harbors 23 positively charged protons, balanced by an equal number of negatively charged electrons.
Electron Configuration: A Blueprint of Energy Levels
Electrons, the indefatigable inhabitants of atoms, reside in designated energy levels known as orbitals. The arrangement of these electrons, termed the electron configuration, provides a roadmap to the element’s chemical behavior. Vanadium’s electron configuration is beautifully represented as [Ar] 3d³ 4s².
Breaking Down the Electron Configuration of Vanadium
To decode this seemingly complex notation, we dive deeper into the electronic realm of vanadium. The [Ar] prefix signifies that vanadium’s inner-shell electrons mirror the stable arrangement of the noble gas argon (18 electrons). This leaves us with just three electrons in the outermost energy level, known as the valence shell: two in the 4s orbital and one in the 3d orbital.
Valence Electrons: The Key Players in Chemical Bonding
Valence electrons, the dynamic occupants of the outermost shell, play a pivotal role in determining an element’s chemical personality. They are the gatekeepers of chemical bonding, eagerly seeking companionship to form stable compounds. Vanadium’s three valence electrons, eager for interaction, make it a versatile participant in a multitude of chemical reactions.
Our quest to unravel the secrets of vanadium’s electron configuration has led us to a profound understanding of its atomic structure and chemical potential. Vanadium, with its unique combination of atomic number, electron configuration, and valence electrons, stands as a testimony to the complex and captivating world of chemistry.
Unveiling the Mystery of Vanadium’s Valence Electrons
In the realm of chemistry, vanadium stands out as a captivating element with exceptional properties. One of the fundamental questions that intrigues scientists and students alike is: “How Many Valence Electrons Does Vanadium Possess?” Embark on a journey to unravel this mystery as we delve deeper into the fascinating world of vanadium’s electron configuration.
Atomic Number: A Gateway to Understanding
The atomic number of an element serves as a cornerstone in deciphering the number of protons and electrons it harbors. For vanadium, this atomic number is 23, revealing that it boasts 23 protons and 23 electrons.
Electron Configuration: A Blueprint of Electronic Distribution
Electron configuration, the arrangement of electrons within an atom’s orbitals, provides valuable insights into an element’s chemical behavior. Vanadium’s electron configuration, written as [Ar] 3d³ 4s², unveils its electronic blueprint. This configuration indicates that vanadium has:
- 3 electrons in the 3d orbital
- 2 electrons in the 4s orbital
Valence Electrons: The Key Players in Chemical Bonding
In the realm of chemistry, valence electrons play a pivotal role, acting as the ambassadors that facilitate interactions between atoms. These electrons reside in the outermost energy level of an atom, eager to participate in chemical bonding. Vanadium boasts 5 valence electrons, consisting of:
- 3 electrons in the 3d orbital
- 2 electrons in the 4s orbital
These valence electrons endow vanadium with the capacity to form chemical bonds with other elements, enabling it to participate in a wide array of reactions and compounds. Their significance lies in their ability to determine an element’s reactivity and the types of bonds it can form.
Through this exploration, we have unraveled the mystery of vanadium’s valence electrons:
- Vanadium, with an atomic number of 23, possesses 23 protons and 23 electrons.
- Its electron configuration, [Ar] 3d³ 4s², reveals the presence of 3d³ electrons and 4s² electrons.
- Vanadium boasts 5 valence electrons, 3 in the 3d orbital and 2 in the 4s orbital.
- These valence electrons play a crucial role in vanadium’s chemical bonding capabilities, influencing its reactivity and the formation of compounds.
By understanding the number and distribution of valence electrons, we gain a deeper appreciation of vanadium’s unique properties and its ability to contribute to the vast tapestry of chemical reactions. As we continue to explore the world of elements, may this journey serve as a reminder that even the smallest of particles hold secrets that unlock the wonders of chemistry.