Understanding Valence Electrons: A Guide To Sodium (Na) And Its Chemical Properties
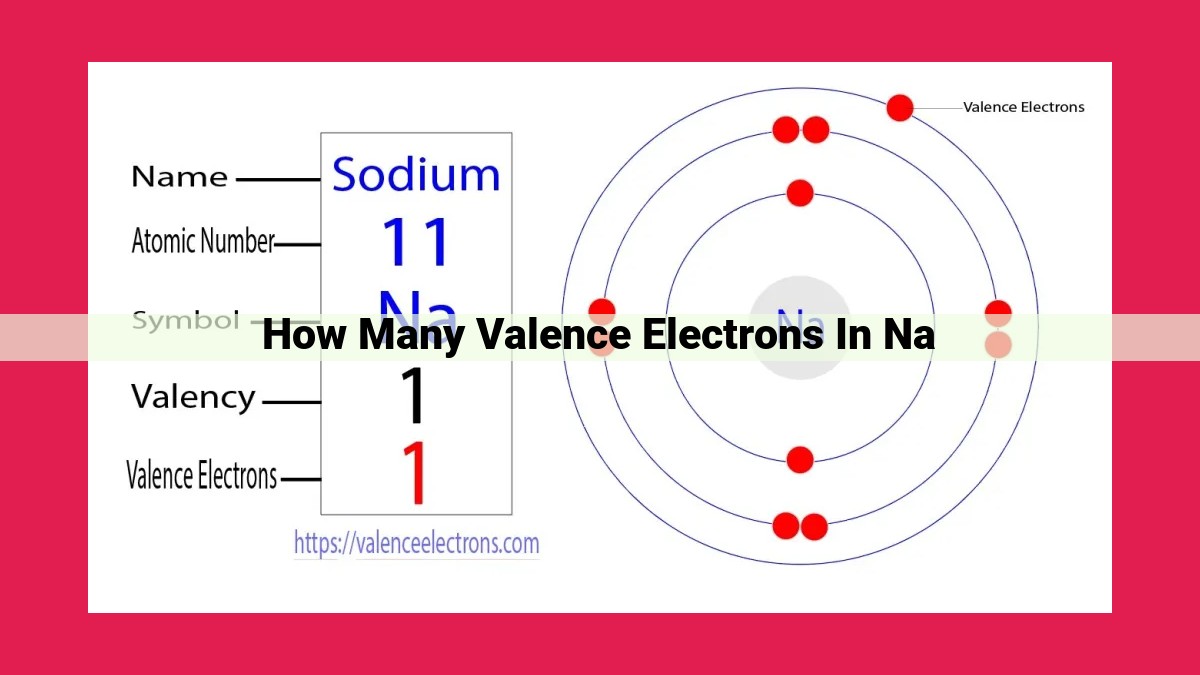
Sodium (Na) has 1 valence electron. The number of valence electrons is determined by the electron configuration of an element, which indicates the arrangement and distribution of electrons in its atomic orbitals. Na has 11 electrons, with an electron configuration of [Ne] 3s¹ where Ne represents the noble gas Neon and 3s¹ represents the single valence electron in the outermost s orbital. Valence electrons play a critical role in chemical reactions, as they determine an element’s chemical properties and its ability to form bonds with other atoms or molecules.
Delving into the Realm of Valence Electrons
Valence electrons, like the enigmatic stars in the chemical cosmos, hold the key to unlocking the secrets of chemical reactions. They are the electrons that reside in the outermost energy level of an atom, eager to dance with their counterparts to form the very building blocks of our world.
Their Enigmatic Influence
The significance of valence electrons cannot be overstated. They determine an atom’s chemical reactivity, its tendency to interact with other atoms. This reactivity is the driving force behind the formation of chemical bonds, the invisible threads that bind atoms together to create molecules and compounds.
By understanding the dance of valence electrons, we can unravel the mysteries of chemical reactions, predict the properties of substances, and design materials with tailored characteristics. They are the gateway to manipulating the atomic realm, unlocking the potential for transformative innovations.
Uncovering the Secrets of Sodium
Let’s take a closer look at the enigmatic sodium atom, a testament to the power of valence electrons. Sodium’s electron configuration, the arrangement of its electrons, is 1s² 2s² 2p⁶ 3s¹. This intricate pattern reveals that sodium has one valence electron in its outermost 3s orbital.
This lone valence electron is the key to sodium’s chemical reactivity. It eagerly seeks a partner to form a stable duet, making sodium a highly reactive metal. In chemical reactions, it readily donates its valence electron to achieve the coveted noble gas configuration, a stable electron arrangement resembling the noble gases.
The Symphony of Atomic Structure
The placement of valence electrons is intimately connected to the atomic structure. Atoms strive to achieve the stable electron configuration of the noble gases, elements like helium and argon. This quest for stability dictates the number of valence electrons an atom possesses.
Periodic Patterns
As we traverse the periodic table, we observe distinct periodic trends in valence electron configurations. Elements in the same group share the same number of valence electrons, reflecting the arrangement of their energy levels. This understanding enables us to predict the chemical behavior of elements based on their position in the periodic table.
The Octet Rule
For main group elements, the octet rule shines as a guiding principle. It states that these elements tend to have eight valence electrons, either by gaining or losing electrons to achieve a noble gas-like configuration. This rule helps explain the formation of stable chemical bonds and the properties of compounds.
Valence Electrons in Sodium: Understanding the Key to Chemical Reactions
In the vast world of chemistry, the concept of valence electrons plays a pivotal role, shaping the behavior and properties of elements. These electrons, located in the outermost energy level of an atom, hold the key to understanding how elements interact with each other to form molecules and compounds.
Electron Configuration of Sodium
Let’s take sodium (Na) as an example. This alkali metal, with its atomic number 11, boasts an electron configuration of 1s²2s²2p⁶3s¹. In this configuration:
- The superscript numbers indicate the energy levels, with 1 being the innermost and 3 being the outermost.
- The lettered subscripts (s, p) represent the sublevels within each energy level.
Determining Valence Electrons in Sodium
To determine the number of valence electrons in sodium, we focus on the outermost energy level, which is 3s¹. This outermost sublevel contains only one electron. Therefore, sodium has one valence electron.
Valence Electrons and Chemical Behavior
The number of valence electrons is crucial for understanding chemical behavior because these electrons are the ones involved in forming chemical bonds. In the case of sodium, its single valence electron makes it highly reactive. This reactivity stems from sodium’s desire to achieve a stable electron configuration by losing its valence electron and becoming a positively charged ion.
Atomic Structure and the Noble Gas Configuration
At the heart of every atom lies a fascinating dance of electrons. Among these electrons, valence electrons take the lead, determining the atom’s chemical character. Understanding their role in atomic structure unravels the secrets behind chemical reactions and the behavior of elements.
The number of valence electrons in an atom can be predicted by its position on the periodic table. Elements in the same group share the same number of valence electrons, giving them similar chemical properties. For instance, sodium (Na), an alkali metal, has one valence electron.
This valence electron is perched on the outermost shell of the sodium atom, far from the nucleus’s pull. Due to its loose attachment, it can easily break free during chemical reactions, making sodium highly reactive.
Achieving the noble gas configuration is a celestial dance that every atom aspires to. Noble gases, such as helium (He), neon (Ne), and argon (Ar), are paragons of stability. They have eight valence electrons, forming a complete and stable outer shell.
Likewise, atoms strive to attain this stable octet of valence electrons. When an atom acquires or loses electrons, it undergoes a chemical transformation until it mirrors the inert noble gas configuration. This relentless pursuit drives chemical reactions, forging bonds between atoms to achieve the ultimate electronic balance.
Valence Electrons in the Periodic Table
In the realm of chemistry, the concept of valence electrons unravels a captivating narrative that sheds light on the enigmatic world of chemical reactions and atomic interactions. These pivotal electrons reside in the outermost energy level of an atom and play a pivotal role in determining the atom’s chemical reactivity.
As we embark on a journey through the periodic table, we uncover fascinating patterns in the configurations of valence electrons. For instance, the elements in the main group (also known as the representative elements), which occupy the columns from 1 to 8, exhibit a characteristic number of valence electrons.
Alkali metals, such as sodium (Na), lithium (Li), and potassium (K), possess a single valence electron, residing in an outermost shell of two electrons. This lone valence electron yearns for a dance partner, making these elements highly reactive and eager to form chemical bonds with other atoms.
In contrast, halogens, like fluorine (F), chlorine (Cl), and bromine (Br), have seven valence electrons. Their electron configurations are just one electron shy of achieving the coveted octet rule, a stable and inert state. This atomic craving drives halogens to readily accept electrons, forming stable compounds with alkali metals.
As we traverse the periodic table horizontally, from left to right, the number of valence electrons increases. This trend underscores the increasing number of electron shells as we move across the rows. Consequently, the chemical reactivity of nonmetals decreases, as they seek fewer electrons to complete their stable octet.
Main group elements strive to attain the noble gas configuration, a highly stable and unreactive arrangement with eight valence electrons. This configuration renders noble gases, such as helium (He) and neon (Ne), chemically inert.
The concept of valence electrons is a fundamental pillar of chemistry, offering profound insights into the intricate dance of atoms and molecules. By understanding the presence and configuration of valence electrons, we can unravel the secrets of chemical reactions and predict the behavior of elements within the periodic table.
Valence Electrons and Chemical Bonding: The Key to Unlocking Chemical Reactions
In the realm of chemistry, understanding the behavior of elements is crucial for unraveling the mysteries of chemical reactions. One fundamental aspect that plays a central role in this understanding is the concept of valence electrons. These electrons, residing in the outermost energy level of an atom, hold the key to chemical bonding, the process that unites atoms to form molecules and compounds.
The Dance of Ions: Ionic Bonding
When valence electrons are playful and adventurous, they can embark on a journey to explore the world outside their home atom. Sometimes, they encounter atoms with an insatiable hunger for electrons, like sodium (Na). These atoms, eager to complete their electron dance, willingly give up their valence electron.
This transfer of electrons creates an electrical imbalance, resulting in the formation of ions. The electron donor, sodium, transforms into a positively charged ion (Na+), while the recipient atom becomes negatively charged. Opposites attract, and these oppositely charged ions are drawn together by an irresistible force, forming an ionic bond.
The Embrace of Covalence: Covalent Bonding
But not all valence electrons are as daring as those in sodium. Some prefer to forge deeper connections, sharing their dance with other atoms to create a harmonious bond. This dance of shared electrons gives birth to covalent bonds.
In covalent bonding, valence electrons from two or more atoms overlap, forming a cloud of electron density that envelops the atoms. This shared electron cloud acts like a strong glue, holding the atoms together in a covalent molecule.
The Guiding Principle: The Octet Rule
The behavior of valence electrons is guided by a principle known as the octet rule. This rule dictates that atoms strive to achieve a stable electron configuration with eight valence electrons. It’s like the feng shui of electron arrangements, and it’s essential for understanding chemical bonding.
Atoms with complete octets, like the noble gases, are content and rarely react. But atoms with incomplete octets yearn for stability and actively seek to complete their electron configuration. This desire drives chemical reactions and determines how elements interact with each other.
Valence electrons are the architects of chemical bonding, the invisible dance that brings atoms together. Their ability to waltz between atoms or embrace in a shared embrace determines the formation of ionic and covalent bonds. Understanding the role of valence electrons is the key to unlocking the mysteries of chemical reactions and predicting the behavior of elements in the vast world of chemistry.