Valence Electrons Of Palladium: Unlocking Chemical Versatility For Research And Industry
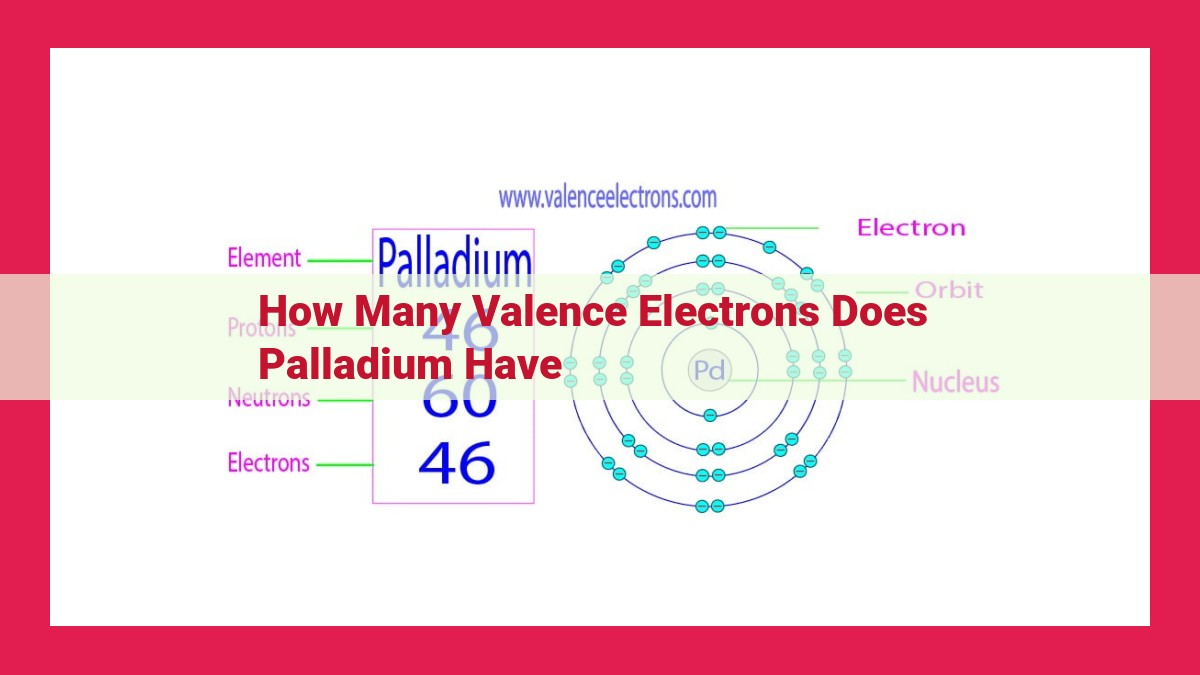
Palladium possesses 10 valence electrons, residing in its d-orbitals. These valence electrons determine its chemical behavior and versatility. The absence of s-orbital valence electrons and the presence of these d-orbital electrons contribute to palladium’s unique properties. Understanding the valence electron configuration of palladium is crucial for comprehending its chemical bonding and reactivity, making it a significant aspect in various research and industrial applications.
Palladium: Unveiling the Secrets of Valence Electrons
In the realm of chemistry, valence electrons hold the key to understanding the fascinating world of chemical properties. These essential electrons, located in the outermost energy level of an atom, dictate how elements interact and form bonds with each other.
In this captivating exploration, we delve into the mysterious world of palladium, a precious metal renowned for its versatile nature. With an atomic number of 46, palladium boasts a unique electron configuration that reveals its remarkable chemical properties.
Our journey begins by uncovering the significance of valence electrons in determining the chemical behavior of elements. These elusive electrons are responsible for the formation of chemical bonds, the driving force behind the countless reactions that shape our world. As we explore the intriguing case of palladium, we will unravel the secrets of its valence electrons and discover how they contribute to its exceptional chemical versatility.
Electron Configuration and Valence Electrons
Palladium, an element of great interest in the realm of chemistry, possesses a unique electron configuration that shapes its remarkable properties. With an atomic number of 46, palladium boasts a total of 46 electrons distributed across its various orbitals.
The heart of palladium’s electron configuration lies in its valence electrons, which are the outermost electrons that determine its chemical reactivity. Palladium’s valence electrons reside exclusively within its d-orbitals, marking a departure from the typical presence of valence electrons in both s- and d-orbitals. This absence of s-orbital valence electrons in palladium is a distinctive feature that sets it apart.
Palladium’s d-orbital valence electrons, numbering 10 in total, occupy the 4d orbitals. These orbitals, unlike the spherical s-orbitals, possess unique shapes resembling four-lobed “cloverleaves.” The 4d orbitals in palladium are oriented in specific directions, contributing to the element’s overall electron configuration and its reactivity.
The d-orbital valence electrons play a pivotal role in palladium’s chemical bonding, endowing it with remarkable versatility. These electrons engage in a range of bonding interactions, forming stable and complex compounds. Palladium’s ability to participate in diverse chemical bonds stems from the availability of its d-orbital valence electrons.
In summary, the electron configuration of palladium is characterized by the absence of s-orbital valence electrons and the presence of 10 d-orbital valence electrons. This unique configuration leads to the formation of 4d orbitals with intriguing shapes and orientations. These d-orbital valence electrons are the driving force behind palladium’s versatile chemical bonding capabilities, making it an element of great interest in both scientific research and practical applications.
Atomic Orbitals and Valence d Electrons in Palladium
Each element’s unique chemical properties are determined by the arrangement of its electrons, particularly those in the outermost shell called valence electrons. In this article, we’ll dive into the fascinating world of valence electrons in palladium and explore their profound impact on its chemical behavior.
Atomic Orbitals: The House for Electrons
Imagine electrons as tiny particles dancing around the nucleus of an atom. Each electron occupies a specific atomic orbital, a region of space where it is most likely to be found. Orbitals come in various shapes, just like the rooms in a house, and have different orientations, just like the arrangement of furniture in a room.
The Unique 4d Orbitals in Palladium
Palladium, with an atomic number of 46, has 10 valence electrons in its outermost shell. These valence electrons reside in 4d orbitals, which have intricate and unique shapes. Unlike the spherical s orbitals or dumbbell-shaped p orbitals, 4d orbitals feature more complex patterns, including cloverleaf-shaped and double-dumbbell-shaped configurations.
The Role of Valence d Electrons in Chemical Bonding
The valence d electrons of palladium are not only fascinating in shape but also highly influential in chemical bonding. These electrons can participate in various bonding interactions, such as:
- Covalent bonds: Sharing electrons with other atoms to form stable molecules.
- Ionic bonds: Transferring electrons to or from other atoms to create charged particles.
- Metallic bonds: Sharing electrons in a sea of delocalized electrons, resulting in high electrical and thermal conductivity.
The versatility of palladium’s valence d electrons enables it to form compounds with diverse structures and properties, making it an essential element in many industrial and catalytic applications.
Chemical Bonding in Palladium
- Describe the different types of chemical bonds that palladium can participate in.
- Explain how valence d electrons contribute to the formation of stable bonds.
- Discuss the chemical versatility of palladium due to its valence electrons.
Chemical Bonding in Palladium: Unlocking the Secrets of Valence Electrons
Just like us humans have unique attributes that shape our lives, the atoms of Palladium have a special characteristic that gives them extraordinary chemical powers – their Valence Electrons.
In the world of chemistry, valence electrons are like the social butterflies of atoms, determining their ability to bond with other atoms and form molecules. Palladium has a knack for attracting these bonding partners with its impressive collection of 10 valence electrons, all residing in its d-orbitals.
The unique 4d orbitals in palladium have a special shape, which allows them to interact with other atoms in a highly flexible manner. This remarkable trait grants palladium the ability to participate in various types of chemical bonds:
-
Strong Metallic Bonds: Palladium atoms can link together through strong metallic bonds, forming a solid, silvery-white metal.
-
Covalent Bonds: Palladium’s valence d electrons make it adept at sharing electrons with other non-metallic atoms, creating covalent bonds. This versatility allows palladium to bond with elements like carbon, nitrogen, and oxygen.
-
Coordination Bonds: Palladium has a special talent for forming coordination bonds with ligands – molecules or ions that have extra electrons. This ability makes palladium indispensable in catalysis, which is the art of speeding up chemical reactions.
The abundance of valence d electrons in palladium gives it unmatched chemical versatility. It can act as a catalyst, promoting reactions between other molecules without being consumed. It can also form stable complexes with a wide range of ligands, making it a valuable player in medicinal chemistry and drug development.
Understanding the role of valence electrons in palladium is crucial for unlocking its potential in fields such as electronics, catalysis, and medicine. These electrons are the key to unraveling the exceptional chemical properties that make palladium a true scientific gem.