Understanding The Role Of Atomic Number In The Properties And Applications Of Uranium

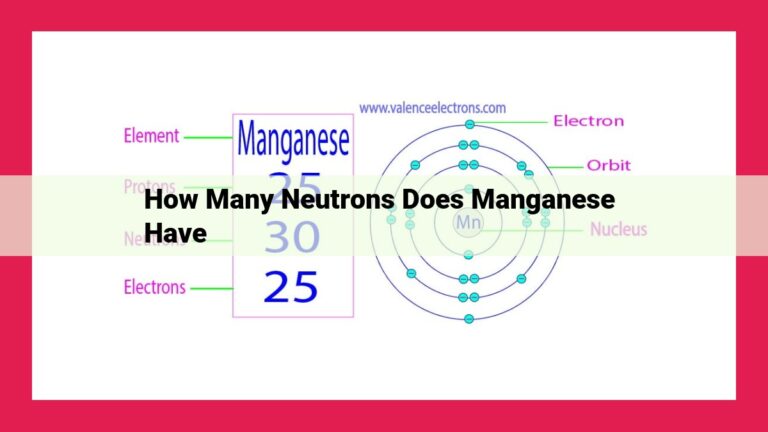
Uranium, an element with atomic number 92, possesses 92 protons within its nucleus. The atomic number defines an element’s identity and characteristics. Uranium’s high atomic weight and metallic appearance, along with its unique radioactive properties, stem from its atomic number. Furthermore, this number determines its applications in nuclear power and medical fields, as it influences its reactivity and energy-generating potential. The atomic number serves as a fundamental guide for understanding uranium’s behavior and its implications in scientific research and technological advancements.
Atomic Number: Unlocking the Secrets of Proton Count
In the realm of atoms, each element possesses a unique fingerprint that defines its characteristics and behavior – the atomic number. Like an invisible gateway, the atomic number grants us access to understanding the number of protons residing in an atom’s nucleus.
Delving into the Atomic Number
The atomic number, a fundamental property of every atom, is defined as the total number of positively charged protons in its nucleus. This numero holds immense significance, as it not only distinguishes one element from another but also dictates the element’s chemical properties.
Unveiling the Proton Mystery
Consider the element uranium, with its atomic number of 92. This numerical label reveals that each uranium atom harbors 92 protons, making it a distinct entity among the elements. The arrangement of these protons within the nucleus shapes uranium’s unique characteristics, including its weight, appearance, and reactivity.
Atomic Number: A Guide to Elemental Behavior
The atomic number acts as a guiding star, illuminating the path to comprehending the properties and behavior of uranium. It governs uranium’s metallic nature, its high atomic weight, and its exceptional radioactive properties. By unraveling the mysteries of atomic number, scientists have harnessed uranium’s energy potential in nuclear power plants and employed its radioactivity for medical advancements.
The atomic number stands as a gateway to understanding the intricate world of atoms and their behavior. It opens doors to discovering the unique characteristics of each element, guiding scientific research and technological advancements. As we delve deeper into the atomic realm, the significance of atomic number as a fundamental building block of elemental identity and properties continues to captivate and inspire.
Uranium’s Atomic Number: Unveiling the Proton Mystery
In the tapestry of elements that weaves the fabric of our universe, uranium stands out as a captivating protagonist with its intriguing properties and profound implications. At the heart of uranium’s enigmatic nature lies its atomic number, a fundamental concept that holds the key to understanding its behavior and unraveling its mysteries.
Defining Atomic Number
The atomic number of an element is the number of protons residing in the nucleus of its atoms. Protons are positively charged subatomic particles that determine an element’s unique identity and characteristics. Uranium’s atomic number is 92, signifying that each atom of uranium contains a resolute garrison of 92 protons within its nucleus.
Relationship with Elemental Characteristics
The atomic number of an element serves as a crucial compass, guiding us through the element’s behavior and properties. For uranium, its atomic number of 92 molds its destiny, shaping its high atomic weight, which contributes to its substantial density and stability. The 92 protons within uranium’s nucleus also orchestrate its metallic appearance, as they readily shed electrons, granting it a lustrous sheen and malleable nature.
Unveiling Uranium’s Distinctive Traits
Probing deeper into the influence of atomic number, we encounter uranium’s prominent radioactive properties. The 92 protons in uranium’s nucleus create an unstable equilibrium, fostering a tendency for uranium to decay radioactively. This decay process liberates immense energy, making uranium a potent source of nuclear power and a vital component in medical applications like cancer treatment.
**Unveiling Uranium’s Atomic Properties**
The atomic number of an element tells us the number of protons in its nucleus. For uranium, this number is 92, indicating that each uranium atom contains 92 protons. This seemingly simple number holds the key to understanding uranium’s unique properties.
High Atomic Weight and Metallic Appearance
Uranium’s atomic number heavily influences its atomic weight. With 92 protons and 146 neutrons, uranium ranks as one of the heaviest elements naturally occurring on Earth. This massive atomic weight contributes to uranium’s high density and solid, metallic appearance.
Radioactive Properties
But uranium’s atomic number also reveals a more mysterious aspect of the element: its radioactivity. The presence of such a high number of protons in the nucleus makes uranium unstable, leading to the spontaneous decay of atoms through processes like alpha emission and fission. These radioactive properties have both benefits and risks, making uranium a crucial element for nuclear power generation and medical applications.
Implications for Uranium’s Applications
The atomic number of 92 not only shapes uranium’s properties but also dictates its suitability for various applications. In nuclear power plants, uranium’s high atomic weight and radioactive properties enable it to be used as a fuel, releasing immense energy through nuclear fission. In medicine, uranium isotopes find use in targeted therapies and imaging techniques.
Understanding uranium’s atomic properties is fundamental to harnessing its power and mitigating its hazards. As scientists delve deeper into the realm of atomic physics, the insights gained from uranium’s atomic number continue to guide research and technological advancements.
Linking Atomic Number to Uranium’s Applications
Uranium’s atomic number of 92 is not just a mere number; it holds immense significance in unlocking the element’s versatile applications. This atomic number determines uranium’s suitability for various fields, from nuclear power generation to medical advancements.
Nuclear Power Plants: Harnessing Uranium’s Energy
In nuclear power plants, uranium’s high atomic number plays a pivotal role. The 92 protons in its nucleus make uranium an ideal fuel for nuclear reactions. These protons create a strong nuclear force that holds the nucleus together, releasing enormous energy when it is split apart. This process, known as nuclear fission, generates heat that is used to produce electricity.
Medical Applications: Healing with Uranium
Beyond nuclear energy, uranium’s atomic number has also found applications in the medical field. Radioisotopes of uranium, which have slightly different numbers of neutrons than the stable isotope, are used for diagnostic imaging and cancer treatment. Uranium’s atomic number influences its radioactive properties, allowing these radioisotopes to emit specific types of radiation that can be harnessed for medical purposes.
In conclusion, uranium’s atomic number is a crucial factor that shapes its diverse applications. Its high number of protons enables its use in nuclear power generation, while its unique radioactive properties make it valuable in medical advancements. Understanding the significance of atomic number provides a deeper appreciation for the fascinating element of uranium and its contributions to modern society.
Atomic Number: The Guide to Understanding Elemental Behavior
Just as a blueprint dictates the structure of a building, the atomic number serves as a fundamental blueprint for understanding the properties and behavior of elements. It’s like a unique identification number that opens the door to deciphering the characteristics of each element.
The Atomic Number’s Significance
The atomic number, represented by the symbol “Z”, is the number of protons residing within an atom’s nucleus. It’s a crucial factor in determining an element’s identity and its placement on the periodic table. With each increment in atomic number, a new element emerges with distinct properties.
Uranium: A Case Study in Atomic Number
Let’s take uranium, for instance. Its atomic number of 92 reveals that each uranium atom is home to 92 protons in its nucleus. This fundamental attribute governs the element’s characteristics, such as its hefty atomic weight and signature metallic appearance.
Implications for Scientific Discovery
The atomic number acts as a compass for scientific research. By knowing the atomic number of an element, researchers can anticipate its reactivity, energy-generating potential, and other properties. This knowledge has propelled advancements in fields such as nuclear power and medicine.
Uranium’s Applications: A Testament to Atomic Number
Uranium’s atomic number of 92 directly influences its suitability for nuclear power plants. The high number of protons in uranium’s nucleus makes it an ideal candidate for generating energy through nuclear reactions. Similarly, in medical applications, uranium’s radioactive properties, shaped by its atomic number, aid in cancer treatments and diagnostic imaging.
The atomic number is a foundational concept that guides our understanding of the chemical world. It’s a key that unlocks the secrets of elements, enabling scientists and researchers to harness their properties for groundbreaking advancements. As we continue to delve into the mysteries of the atomic realm, the atomic number remains our steadfast companion, illuminating the path to scientific discovery.