Unveiling Similarities Between Elements And Compounds: A Journey Into The Building Blocks Of Matter
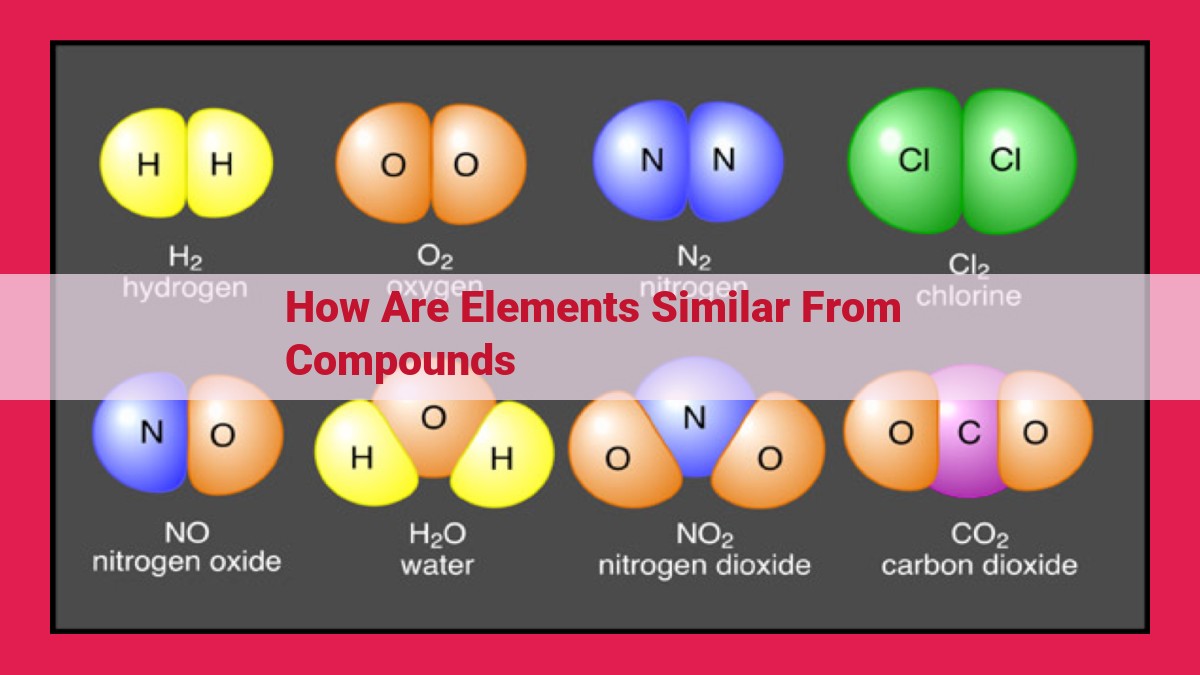
Elements and compounds share fundamental similarities. Both are composed of atoms, with isotopes and atomic masses playing significant roles. They exhibit similar chemical properties, engaging in chemical reactions through various bonding mechanisms. The concept of molecules extends to compounds, allowing for comparisons in terms of molecular weight and formula. Understanding the similarities between elements and compounds is crucial in unraveling the interconnectedness of matter, showcasing how these fundamental building blocks contribute to the diversity of chemical substances.
Definition of Elements and Compounds
- Explain the concept of elements and their organization in the periodic table.
- Define compounds and describe the process of chemical bonding that forms them.
Understanding the Building Blocks: Elements and Compounds
In the vast tapestry of our universe, matter takes on countless forms, from the stars above to the ground beneath our feet. The fundamental components of this intricate world are elements and compounds – the building blocks of everything.
Elements: The Simplest of Forms
Elements stand as the simplest form of matter. They cannot be broken down into simpler substances by chemical means and are represented by their symbols on the periodic table, like H for hydrogen, O for oxygen, and Fe for iron.
Arranged in order of increasing atomic number, the periodic table showcases elements with similar properties grouped in columns, called groups or families. These groups reveal patterns in chemical reactivity and the formation of compounds.
Compounds: A Union of Elements
Compounds are born when two or more elements join together through chemical bonding. This process involves the sharing or exchanging of electrons between atoms, creating new substances with unique properties not found in their individual elements.
For example, when the element sodium (Na) combines with chlorine (Cl), it forms the compound sodium chloride (NaCl), commonly known as table salt. In this union, sodium transfers an electron to chlorine, resulting in a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-), which are held together by ionic bonding.
Similarities in Building Blocks of Matter
- Emphasize that both elements and compounds are composed of atoms.
- Discuss the concept of isotopes and atomic mass.
Similarities in the Building Blocks of Matter
At the heart of all matter, both in the world around us and within our own bodies, lies a common thread connecting all substances. This thread is the atom, the fundamental building block of the universe. Whether we examine elements, the simplest of all substances, or compounds, their intricate combinations, atoms are the fundamental units from which all matter is constructed.
Elements, the purest form of matter, consist of a single type of atom, each with unique properties determined by its number of protons and electrons. These atoms, arranged in an orderly fashion, form the periodic table, a roadmap of all known elements. In contrast, compounds are formed when atoms of different elements combine in specific ratios, forming new substances with properties distinct from their individual components.
Despite their different compositions, both elements and compounds share a fundamental similarity: they are both composed of atoms. These atoms, the basic units of matter, possess a central nucleus, housing protons and neutrons, and a surrounding cloud of electrons. It is the arrangement of these electrons, particularly those in the outermost shell, that defines the chemical properties of an atom.
Another intriguing aspect of atoms is the concept of isotopes. Isotopes are variations of the same element that possess the same number of protons but differ in the number of neutrons. This difference affects the atomic mass of the element, which is the average mass of all its isotopes. Understanding isotopes is crucial in various fields, including chemistry, geology, and nuclear physics, as it provides insights into the composition and properties of matter.
Common Chemical Properties: Exploring the Bonds that Define Matter
In the vast tapestry of chemistry, matter exists in two fundamental forms: elements and compounds. While distinct in nature, they share striking similarities in their chemical properties. Both elements and compounds possess an innate ability to engage in transformative chemical reactions, reshaping their molecular structures and forming new substances.
Just as the elements are organized in the periodic table according to their atomic number, chemical bonds are the architects that govern the formation and behavior of compounds. These bonds, ionic, covalent, and metallic, represent the forces that hold atoms together.
Ionic bonds, marked by the transfer of electrons between atoms, forge compounds between highly reactive metals and nonmetals. The resulting compounds, known as salts, are often characterized by their solubility in water and ability to conduct electricity.
Covalent bonds, on the other hand, involve the sharing of electrons between atoms. This type of bonding is prevalent in compounds formed between nonmetals. The resulting molecules exhibit varying properties, ranging from the volatility of gases to the stability of solids.
Metallic bonds are unique to metals. In these bonds, electrons are not confined to individual atoms but form a “sea” around the positively charged metal ions. This arrangement endows metals with their malleability, ductility, and excellent electrical and thermal conductivity.
Understanding the diverse chemical bonds present in both elements and compounds is crucial for unraveling the complexity of the chemical world. These bonds determine the properties of substances, their reactivity, and their potential applications. From the formation of life-sustaining proteins to the development of advanced materials, chemical bonds play a pivotal role in shaping our understanding of the universe.
Molecular Representation: Uncovering the Building Blocks of Matter
At the heart of chemistry lies the concept of molecules, the fundamental building blocks that shape the world around us. Both elements and compounds, the two primary classifications of matter, possess distinctive molecular characteristics.
Elements: The Simplest Entities
Elements, such as oxygen, hydrogen, and carbon, are the simplest forms of matter. Each element consists of a unique atom, the smallest indivisible unit with a distinct number of protons, neutrons, and electrons. These atoms are arranged in an organized manner within the periodic table, a roadmap that showcases the properties and relationships between elements.
Compounds: Molecular Combinations
Unlike elements, compounds are formed when atoms bond together through chemical reactions. These bonds create molecules, which are the structural units of compounds. Covalent bonds, where atoms share electrons, and ionic bonds, where one atom donates electrons to another, are common types of chemical bonds in compounds.
Molecular Weight and Formula: Describing Compounds
Each molecule of a compound possesses a specific molecular weight, which represents the sum of the atomic weights of all its atoms. The molecular formula of a compound, on the other hand, indicates the types and number of atoms present in a single molecule. These molecular characteristics provide essential information about the composition and properties of compounds.
Interconnections of Matter
The study of elements and compounds reveals their intricate interconnectedness. Both substances are composed of atoms and form molecules through chemical reactions. By understanding the building blocks and molecular representation of matter, we unlock the key to unraveling the complexities of the chemical world.
In essence, molecular representation provides a deeper understanding of the structure of matter at its most fundamental level. It allows us to appreciate the beauty and interconnectedness of the chemical world, where the simplest entities combine to form the intricate tapestry of our universe.
The Interconnectedness of Matter: Unveiling the Similarities Between Elements and Compounds
In the tapestry of the chemical world, elements and compounds dance together, intertwining their properties and sharing a profound interconnectedness. Both are essential building blocks of matter, yet they exhibit unique characteristics that contribute to the intricate fabric of our universe.
Elements, the fundamental components of the periodic table, stand alone as individual atoms, each with its own unique set of properties. Compounds, on the other hand, are formed when atoms chemically bond together, creating new substances with distinct characteristics. Despite their differences, elements and compounds share a remarkable bond, revealing the interconnectedness of matter.
At their core, both elements and compounds are composed of atoms, the indivisible units of matter. These atoms can vary in their mass and number of protons, creating different isotopes of the same element. This diversity of atoms contributes to the variety of elements and compounds found in nature.
Reactivity, a fundamental property of matter, binds elements and compounds together. Chemical reactions are the dance of atoms, where elements and compounds come together to form new substances. The types of chemical bonds that form these new substances, such as covalent bonds or ionic bonds, determine the properties of the resulting compound.
Beyond their chemical reactions, elements and compounds share a common language of molecular representation. Molecules are the structural units of compounds, composed of atoms bound by chemical bonds. The molecular weight and molecular formula of an element or compound provide a shorthand for describing its composition and structure.
Understanding the interconnectedness of elements and compounds is crucial for unraveling the complexity of the chemical world. By grasping the similarities and differences between these two building blocks of matter, we gain a deeper appreciation for the intricate dance of atoms and the profound interconnectedness of all things.