Electron Configuration: Unveiling The Arrangement Of Electrons In Atoms
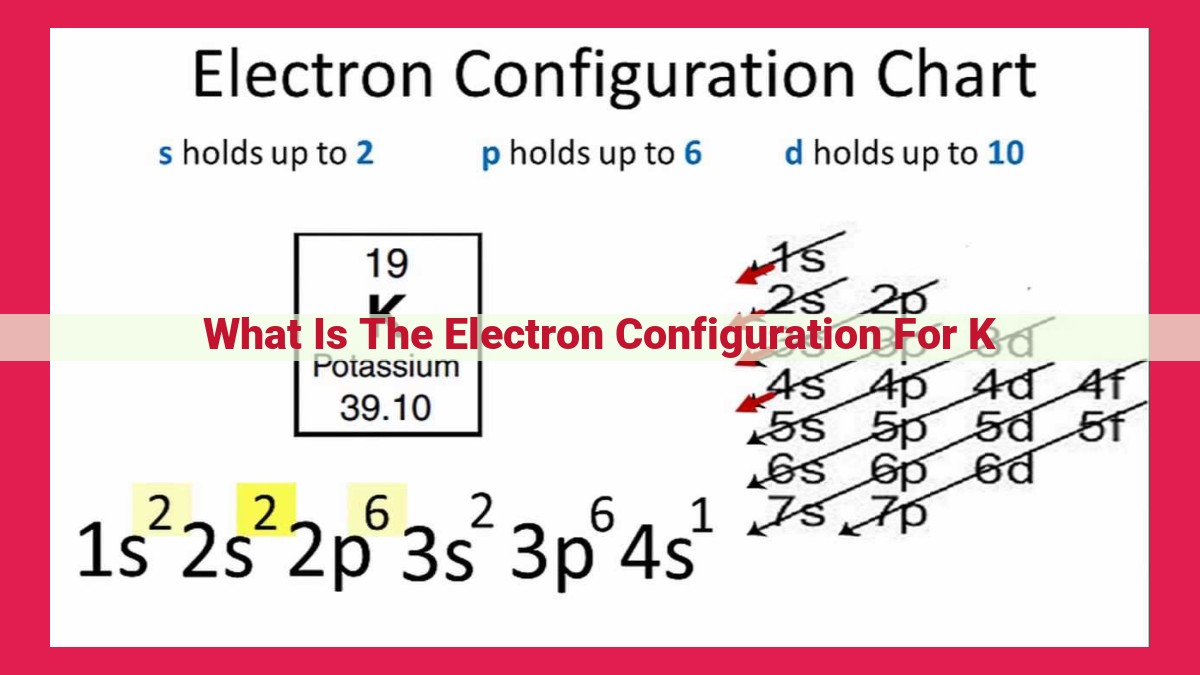
Electron configuration describes the arrangement of electrons in atomic orbitals. The atomic number of an element, representing the number of protons and electrons in the atom, determines its electron configuration. Potassium (K), with an atomic number of 19, has an electron configuration of 1s²2s²2p⁶3s²3p⁶4s¹. This configuration signifies that K has two electrons in the first energy level (1s), eight electrons in the second energy level (2s and 2p), and nine electrons in the third energy level (3s, 3p, and 4s).
- Explain the concept of electron configuration and its importance in understanding element properties.
Every atom that makes up the vast tapestry of our universe holds a hidden blueprint within its nucleus—its electron configuration. This intricate arrangement of electrons dictates the atom’s chemical behavior, shaping its properties and determining its place in the periodic table.
Understanding electron configuration is like deciphering a code that reveals an element’s true identity. It’s a journey into the quantum world, where electrons dance in prescribed orbits, their energy levels and positions defining the atom’s unique characteristics. By unraveling this code, we gain a profound insight into the building blocks of matter.
Atomic Number and Electrons: An Intimate Dance in the Heart of Matter
In the captivating realm of chemistry, the concept of electron configuration plays a pivotal role in unlocking the secrets of element behavior. At its core lies the fundamental understanding of the atomic number, a crucial piece in this intricate puzzle.
Imagine the atomic nucleus as a bustling metropolis, teeming with subatomic particles called protons and neutrons. Each proton carries a positive electric charge, while neutrons remain neutral. The atomic number of an element, denoted by the symbol Z, represents the number of protons residing in its nucleus. This number is unique to each element, acting as its chemical fingerprint.
But here’s where the magic happens: the number of protons in an atom directly corresponds to the number of electrons orbiting it. Electrons, with their negative charge, are the perfect counterbalance to the positive protons, maintaining electrical neutrality in the atom.
Think of it like a delicate dance between partners, where each electron finds its perfect place in the energy levels surrounding the nucleus. These energy levels, like orbital pathways, are organized into concentric shells, each with its own capacity to hold electrons.
As the atomic number increases, so too does the number of electrons in the atom. This gradual addition of electrons influences the element’s properties, its reactivity, and its place in the periodic table. Understanding the relationship between atomic number and electron configuration is the key to unlocking the rich tapestry of chemical behavior.
Electron Configuration of Potassium: Unraveling the Significance of Its Atomic Architecture
In the realm of chemistry, electron configuration plays a pivotal role in understanding the behavior and properties of elements. Electron configuration refers to the arrangement of electrons in an atom’s energy levels. This arrangement governs various chemical characteristics, such as reactivity, bonding, and stability.
One element that showcases a unique electron configuration is Potassium (K). With an atomic number of 19, potassium possesses 19 electrons, orbiting its nucleus in a hierarchical manner. The electron configuration of potassium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
This configuration reveals that potassium has two electrons in the outermost energy level (4s). This lone electron, known as a valence electron, plays a crucial role in determining potassium’s chemical properties.
The valence electron of potassium is loosely bound to the nucleus, making it highly reactive. It readily participates in chemical reactions, readily forming bonds with other atoms to achieve a stable configuration. This reactivity makes potassium a valuable metal in various industries, including fertilizers and explosives.
Moreover, the specific electron arrangement of potassium contributes to its characteristic properties. Potassium is a soft, silvery-white metal with a low melting point. The lone valence electron facilitates the formation of metallic bonds, which explains its malleability and ductility.
In summary, the electron configuration of potassium (1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹) highlights the significance of the valence electron in shaping its chemical behavior. The presence of a single valence electron in the outermost energy level makes potassium highly reactive and endows it with distinctive physical and chemical properties.
Unveiling the Secrets of Electron Configuration: A Key to Understanding Elemental Properties
In the captivating world of chemistry, the electron configuration of an element holds the secrets to unlocking its unique properties. This concept, which describes the arrangement of electrons within an atom, serves as a fundamental pillar in understanding how elements behave.
At the heart of electron configuration lies the atomic number, which determines the number of protons in an atom’s nucleus. This number also corresponds to the number of electrons the atom possesses, since atoms strive to maintain a neutral electrical charge by balancing the positive charges of protons with the negative charges of electrons.
For instance, consider potassium (K), an alkali metal with an atomic number of 19. Potassium’s electron configuration can be expressed as 1s²2s²2p⁶3s²3p⁶4s¹. This intricate configuration signifies the distribution of its 19 electrons across various energy levels, known as orbitals. The 1s orbital holds 2 electrons, the 2s and 2p orbitals hold 2 and 6 electrons respectively, and the 3s, 3p, and 4s orbitals contain 2, 6, and 1 electron respectively.
To delve deeper into electron configuration, we must explore several related concepts:
-
Atomic Mass: This value represents the average mass of an element’s atoms, including the mass of protons, neutrons, and electrons. Isotopes, atoms with the same number of protons but different numbers of neutrons, contribute to the variations in an element’s atomic mass.
-
Noble Gas Configuration: Noble gases, like helium (He) and neon (Ne), possess a stable electron configuration with a full outermost energy level. This configuration lends them exceptional stability, making them unreactive in most chemical processes.
-
Subshells: Energy levels are further divided into subshells, which can hold a specific number of electrons. The s, p, d, and f subshells have capacities of 2, 6, 10, and 14 electrons, respectively.
-
Aufbau Principle: This principle guides the filling of energy levels and subshells. Electrons occupy orbitals in the order of increasing energy, starting with the lowest energy level and subshell.
-
Pauli Exclusion Principle: This principle prohibits any two electrons from having the same set of quantum numbers, which include energy, spin, and orbital shape.