Unveiling Chemical Reactions: A Guide To Transformation, Conservation, And Energy Exchange
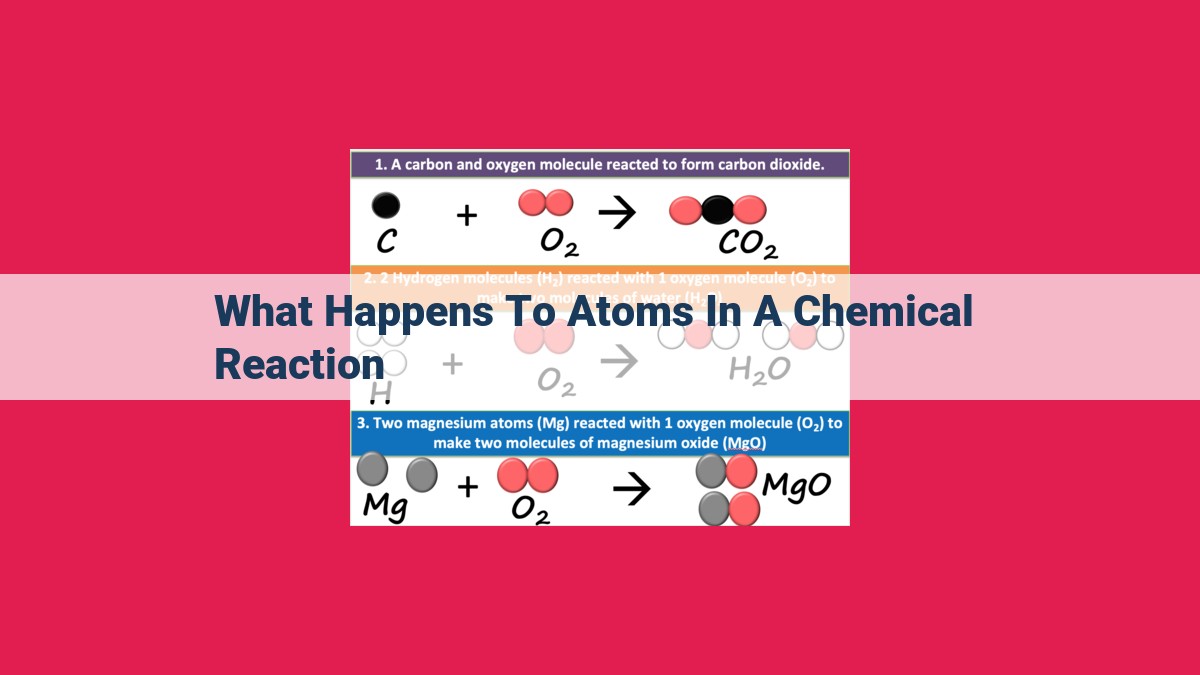
In a chemical reaction, atoms undergo a series of transformations. Reactants, the initial substances, break apart as bonds between their atoms are disrupted. These atoms then rearrange and form new bonds, creating products, the final substances. Chemical reactions involve both the breaking and formation of chemical bonds, governed by the principles of conservation of mass and energy. Throughout the process, the total number of atoms remains unchanged, while energy may be released or absorbed, driving the reaction.
What Happens to Atoms in a Chemical Reaction:
- Definition of a chemical reaction and its significance.
What Happens to Atoms in a Chemical Reaction?
In the realm of chemistry, a chemical reaction is a fascinating dance of atoms and molecules, a transformative process where substances undergo a remarkable change. Understanding the fate of atoms during this dance provides profound insights into the intricacies of chemical transformations.
At the heart of every chemical reaction lie the reactants, the starting materials that embark on a journey of transformation. Reactants are like puzzle pieces, each containing unique atoms and molecular arrangements. As the reaction unfolds, these puzzle pieces interact, their bonds breaking and reforming to create an entirely new set of substances known as products.
The products are the end result of the chemical dance, the new compounds that emerge from the interplay of the reactants. The relationship between reactants and products is governed by the Law of Conservation of Mass, ensuring that the total mass of the reactants equals the total mass of the products. This principle attests to the unwavering balance of nature, where atoms are neither created nor destroyed but merely rearranged.
Chemical reactions are orchestrated by the invisible forces of chemical bonds, the glue that holds atoms together. During a reaction, bonds are broken, separating atoms, and new bonds are formed, connecting atoms in novel arrangements. This delicate interplay of bond breaking and bond formation drives the transformation of reactants into products.
The energy released or absorbed during a chemical reaction is another pivotal aspect. The Law of Conservation of Energy dictates that energy cannot be created or destroyed, only transferred or transformed. In exothermic reactions, energy is released, often in the form of heat or light. In endothermic reactions, energy is absorbed, often in the form of heat or light.
Through the lens of chemical reactions, we witness the dynamic nature of atoms, their ability to rearrange and recombine to form new substances with entirely different properties. By understanding the intricate dance of atoms, we gain a profound appreciation for the transformative power of chemistry and the boundless possibilities it holds for shaping our world.
Reactants: The Starting Point of Chemical Reactions
In the thrilling realm of chemical reactions, reactants take center stage as the initial actors who set the stage for a molecular transformation. These are the substances that enter the chemical arena, poised to embark on a journey of change.
Every chemical reaction is a concerted dance of atoms and molecules. Reactants, the initial cast of this molecular play, bring their own unique atomic arrangements, ready to be rearranged and recombined into new substances. They are the starting point, the raw materials that will undergo a series of chemical transformations.
Reactants can be simple substances, such as hydrogen and oxygen, or they can be complex molecules, like glucose or proteins. Their diversity reflects the vast array of chemical reactions that are possible. However, regardless of their complexity, reactants play a crucial role in determining the outcome of a reaction.
The reactants of a chemical reaction are depicted on the left side of a chemical equation, separated by a plus sign. For example, the equation for the combustion of methane, a key reaction in burning natural gas, reads:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation, CH₄ and 2O₂ represent the reactants. They are the starting point of the reaction, the substances that will undergo a transformation. By understanding the nature of the reactants, we can gain insights into the potential products and the mechanisms by which the reaction will proceed.
Reactants are essential components of chemical reactions, providing the building blocks for the formation of new substances. As the curtain rises on a chemical transformation, the reactants take their place, ready to play their pivotal roles in the molecular drama that unfolds.
Products: The End Result
In the realm of chemical reactions, where atoms engage in a dance of transformation, the products emerge as the tangible outcomes of this molecular waltz. These substances, born from the rearrangement of atomic building blocks, embody the end result of a chemical transformation.
Just as reactants are the raw materials that initiate the reaction, products represent the culmination of atomic interactions. Their existence is inextricably linked to the reactants that gave them birth. In a chemical equation, products are typically written on the right-hand side, signaling their emergence as the consequence of the reaction.
Understanding the nature of products is essential for unraveling the intricate tapestry of chemical reactions. By examining the products formed, chemists can infer the nature of the reaction, the types of atoms involved, and the chemical bonds that govern their interactions. In a combustion reaction, for instance, the product carbon dioxide reveals the presence of carbon and oxygen and the formation of covalent bonds between them.
The relationship between reactants and products is not merely one of cause and effect. In fact, the stoichiometry of a reaction—the quantitative relationship between the reactants and products—provides crucial information about the reaction’s efficiency and the exact amounts of substances involved. This stoichiometric balance ensures that atoms are neither created nor destroyed during the transformation, upholding the fundamental principle of conservation of mass.
In essence, products serve as the final chapter in the story of a chemical reaction. They embody the tangible evidence of atomic transformations and provide valuable insights into the intricate dance of atoms that governs our chemical world.
Chemical Bonds: The Glue Holding Matter Together
In the fascinating realm of chemistry, chemical bonds play a pivotal role in shaping the world around us. They are the invisible forces that hold atoms together, determining the properties of substances and driving the countless chemical reactions that occur in our universe.
Chemical bonds arise from the fundamental nature of atoms, which are composed of positively charged protons and negatively charged electrons. When atoms interact, they strive to achieve a stable electron configuration, which is typically eight electrons in the outermost energy level. This quest for stability leads to the formation of chemical bonds.
There are various types of chemical bonds, each with its unique characteristics. One common type is the covalent bond, which forms when atoms share electrons to achieve a full outermost energy level. For instance, in a molecule of methane (CH4), the carbon atom shares four electrons with four hydrogen atoms, resulting in a stable and nonpolar molecule.
Another type of bond is the ionic bond, which occurs when one atom transfers electrons to another, creating positively and negatively charged ions. For example, in sodium chloride (NaCl), the sodium atom transfers an electron to the chlorine atom, forming positively charged sodium ions and negatively charged chloride ions. These oppositely charged ions then attract each other, forming an ionic bond.
Chemical bonds not only hold atoms together but also influence the reactivity of substances. The strength and type of bonds can determine how easily a substance can undergo chemical reactions. For instance, substances with weak bonds are more likely to react than those with strong bonds.
In a chemical reaction, bonds are broken and formed as atoms rearrange themselves to form new substances. During this process, energy is either absorbed or released, depending on the bonds involved. These energy changes are responsible for the heating or cooling effects we observe in chemical reactions.
Understanding chemical bonds is fundamental to comprehending the behavior of matter and the countless chemical processes that occur in our everyday lives. From the formation of molecules to the reactions that power our bodies, chemical bonds are the driving force behind the chemical world.
Breaking Chemical Bonds: Separating Atoms
In the realm of chemistry, a chemical bond is like a strong handshake between atoms, holding them together in molecules or compounds. When it’s time for a chemical reaction to occur, these bonds must be broken to make way for new ones.
Picture this: You have a necklace with beautiful beads strung together on a chain. To change the beads, you first need to break the chain at the clasp, right? In a chemical reaction, breaking bonds is just like that. It’s a necessary step to separate atoms and make them available to form new bonds.
So, how do atoms break up? There are many ways, but two common methods are:
- Heat: When substances are heated, their atoms gain energy and start moving more vigorously. As they collide, the force can be enough to break the bonds holding them together.
- Light: Certain types of light, like ultraviolet light, carry a lot of energy. When this light strikes a molecule, it can provide the activation energy needed to break bonds and trigger a reaction.
Once bonds are broken, the atoms are free to reorganize themselves into new arrangements. This is where the magic of chemistry happens, as the products of the reaction take shape. Just like building a new necklace by putting together different beads, broken bonds allow atoms to combine in new ways, creating new substances with unique properties.
It’s important to note that breaking bonds doesn’t mean atoms disappear. They simply rearrange, forming new connections and giving birth to new molecules. So, the next time you witness a chemical reaction, remember the vital role of bond breaking. It’s the first step in a transformative journey where atoms dance, break up, and reunite to create the wonders of our chemical world.
Formation of Chemical Bonds: Connecting Atoms
In the realm of chemical reactions, the dance between atoms is not merely a separation or rearrangement but also a profound union. As chemical bonds gracefully form, they connect atoms, giving birth to new substances with unique properties.
This process of bond formation is driven by the relentless pursuit of stability. Atoms, like celestial bodies, seek harmony, and they achieve this balance by sharing or exchanging electrons. When these subatomic particles unite, they create a covalent bond, a strong, yet delicate embrace that holds atoms together.
In a covalent bond, electrons are no longer confined to individual atoms but roam freely within a shared space, creating a molecular orbital. This shared existence enhances the stability of the molecule, reducing its energy and making it less reactive.
Bond formation is not limited to covalent bonds. Ionic bonds, another significant type of chemical bond, arise when one atom donates an electron to another, creating oppositely charged ions. These ions, drawn together by electrostatic forces, form an ionic bond, a bond that often results in the formation of ionic compounds, such as sodium chloride (NaCl).
The formation of chemical bonds is a crucial step in any chemical reaction. It is through these bonds that atoms transform from disconnected entities into cohesive molecules, with each bond serving as a bridge connecting the building blocks of matter. By uniting atoms, these bonds give rise to the vast array of substances that make up our world.
Conservation of Mass: Equal In, Equal Out
In the intricate world of chemical reactions, where atoms dance and rearrange, a fundamental principle reigns supreme: conservation of mass. This law states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products. It’s like a cosmic scale, balancing the ingredients and outcomes with unerring precision.
Consider a hypothetical reaction where sodium and chlorine combine to form sodium chloride, commonly known as table salt. Imagine tiny atoms of sodium, like energetic children, colliding with equally spirited chlorine atoms. Before the reaction, we measure the mass of the sodium and chlorine separately.
As these atoms interact, their electrons mingle and dance, forming new chemical bonds. The once-independent atoms now join forces, creating sodium chloride molecules. Intriguingly, the mass of the newly formed sodium chloride is precisely equal to the combined mass of the original sodium and chlorine atoms. It’s as if the reaction merely reshuffles the atoms, preserving their overall mass.
The principle of conservation of mass is a cornerstone of chemistry, ensuring that no atoms are lost or gained during a reaction. It serves as a constant reminder that matter cannot be created or destroyed, only transformed into different forms. This fundamental law provides a solid foundation for understanding the intricate symphony of chemical reactions that shape our world.
Conservation of Energy: No Loss, No Gain in Chemical Reactions
In the realm of chemistry, the concept of conservation of energy plays a pivotal role in understanding the transformations that occur during chemical reactions. This principle asserts that the total energy of a closed system remains constant, meaning that energy cannot be created or destroyed. In the context of chemical reactions, this translates to the principle that the energy contained in the reactants (the starting materials) is equal to the energy stored in the products (the resulting substances).
This principle has profound implications for our understanding of chemical reactions. It means that the energy released during the breaking of chemical bonds in the reactants is exactly balanced by the energy required to form new chemical bonds in the products. This delicate balance ensures that the total energy of the system remains unchanged.
The conservation of energy in chemical reactions has practical applications in various fields, including:
- Thermochemistry: The study of energy changes associated with chemical reactions.
- Chemical engineering: The design and optimization of chemical processes.
- Biochemistry: The study of chemical reactions that occur in living organisms.
Understanding the conservation of energy in chemical reactions is crucial for scientists and engineers alike. It enables us to predict the energy requirements and outputs of chemical processes, design efficient systems, and gain deeper insights into the intricate workings of the chemical world.