Unveiling The Charge Of Tin: Exploring Atomic Structure, Electron Configuration, And Chemical Reactivity
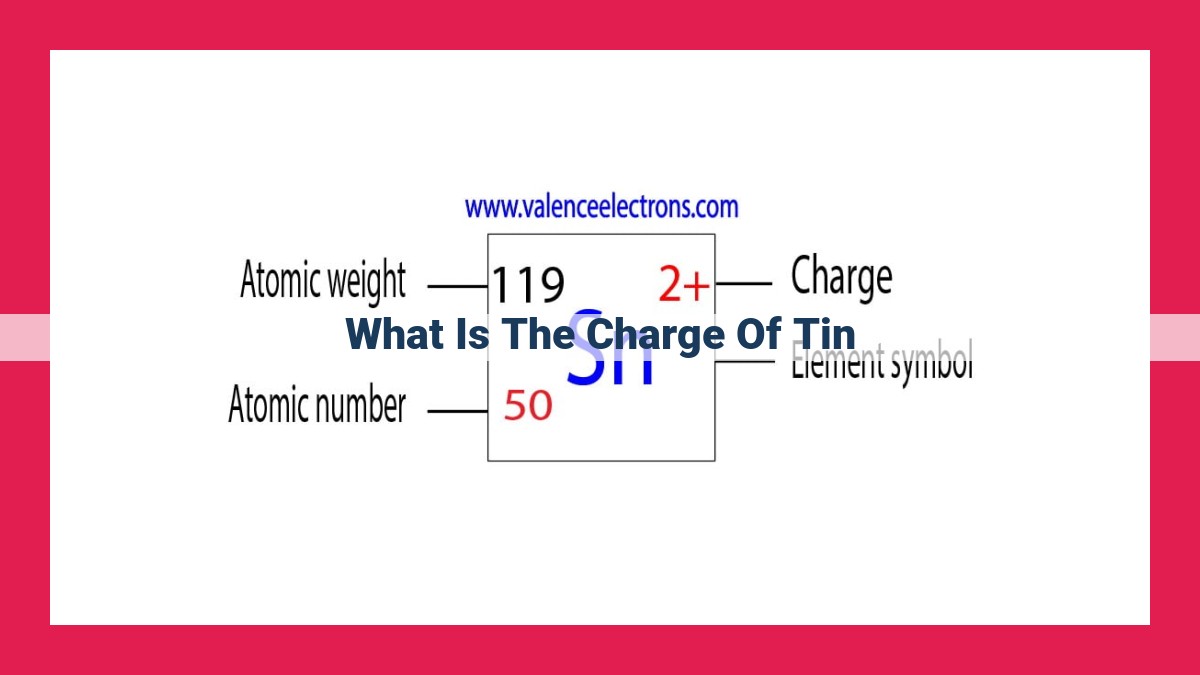
To understand the charge of tin, we explore atomic charge, the atomic number determines the number of protons, which in turn defines the charge of the nucleus. Tin’s atomic number is 50, indicating 50 protons. Electron configuration, with 50 electrons, follows the periodic trends and reveals valency, affecting its chemical bonding and reactivity. Oxidation states and redox reactions involve changes in tin’s charge, and its ionic charge results from electrostatic attraction in ionic bonding. Understanding these concepts provides insights into tin’s chemical and physical properties, with applications in chemistry, metallurgy, and electronics.
Define atomic charge and explain its importance in understanding the chemical and physical properties of tin.
The Significance of Atomic Charge in Understanding Tin’s Chemistry and Properties
In the realm of chemistry, understanding the atomic charge of an element is crucial to unraveling its intricate chemical and physical properties. This charge, which represents the overall balance of protons and electrons in an atom, plays a pivotal role in determining how an element interacts with other atoms and the properties it exhibits.
Take tin, an element that has captivated scientists and industries alike for centuries. Its unique atomic charge not only governs its chemical reactivity but also influences its physical characteristics, such as its malleability and luster.
Tin’s atomic charge is dictated by the number of protons and electrons it possesses. Each proton carries a positive charge, while each electron bears a negative charge. The atomic number of tin is 50, which signifies that each tin atom contains 50 protons. To maintain electrical neutrality, a neutral tin atom also harbors 50 electrons.
The interplay between protons and electrons dictates the electron configuration of an element, which refers to the arrangement of electrons within its atomic orbitals. Tin’s electron configuration, [Kr]4d¹⁰5s²5p², reveals that it has two electrons in its outermost shell, known as valence electrons.
These valence electrons are the key players in determining tin’s chemical reactivity. They participate in chemical bonding by forming bonds with other atoms, either by sharing or transferring electrons. The number and arrangement of valence electrons dictate the valency of an element, which represents its ability to combine with other elements. In the case of tin, its two valence electrons confer upon it a valency of two.
Tin’s atomic charge also manifests in its oxidation states. Oxidation state refers to the apparent charge of an atom in a compound, which is influenced by the number of electrons it gives up or accepts during chemical reactions. Tin exhibits multiple oxidation states, including +2 and +4, reflecting its ability to lose or gain electrons in different chemical contexts.
Furthermore, the atomic charge of tin influences its ionic bonding behavior. When tin atoms lose or gain electrons to achieve a stable electron configuration, they transform into ions. These ions possess an overall positive or negative charge, which enables them to participate in ionic bonding with other ions. For instance, tin(II) ions (Sn²⁺) have lost two electrons, resulting in a +2 charge, while tin(IV) ions (Sn⁴⁺) have lost four electrons, acquiring a +4 charge.
In summary, understanding the atomic charge of an element, such as tin, is paramount in grasping its chemical and physical properties. The atomic charge influences electron configuration, valency, oxidation states, and ionic bonding behavior, ultimately shaping the element’s characteristics and reactivity. This knowledge serves as a cornerstone for scientists and engineers alike, enabling them to harness the unique properties of tin in diverse fields ranging from chemistry to metallurgy and electronics.
Atomic Number and Isotopes: Unraveling the Identity of Tin
The atomic number of an element, like our beloved tin, defines its unique place in the periodic table, acting as its fingerprint amidst the chemical elements. This number reveals the number of protons residing within the atom’s nucleus, the bustling core of the atom. These protons, each carrying a positive electrical charge, determine the overall positive charge of the nucleus.
Complementing the protons are neutrons, their neutral counterparts, also found within the nucleus. Neutrons, as their name suggests, contribute no electrical charge to the nucleus. Together, protons and neutrons collectively contribute to the mass number of an atom, a value that reflects the total number of these subatomic particles.
In the case of tin, its atomic number is 50, indicating the presence of 50 protons in its nucleus. This fundamental characteristic distinguishes tin from all other elements, making it the unique metal we know and utilize.
The mass number of an atom, on the other hand, can vary due to the existence of isotopes. Isotopes are variants of the same element that share the same atomic number but differ in their neutron count. Tin, for instance, has several isotopes, including tin-118, with 68 neutrons, and tin-120, with 70 neutrons. These isotopes have the same number of protons, 50, but different numbers of neutrons, resulting in different mass numbers.
Explain isotopes and how they impact the mass number and charge of tin.
Isotopes and Their Impact on Tin’s Charge
Isotopes: The Building Blocks of Matter
Every element, including tin, is made up of different isotopes. Isotopes are atoms of the same element that have the same atomic number (number of protons) but different numbers of neutrons. This difference in neutrons affects the mass number, which is the sum of protons and neutrons in the nucleus.
Isotopes of Tin
Tin has ten naturally occurring isotopes, with atomic numbers ranging from 112 to 122. The most common isotope is Sn-120, which accounts for about 33% of natural tin. Sn-118 and Sn-119 are also relatively abundant, representing about 24% and 8% of tin, respectively.
Impact on Mass Number and Charge
The number of neutrons in an isotope directly impacts its mass number. Since protons have a positive charge while neutrons are neutral, the overall charge of an isotope remains the same regardless of its neutron count. However, different isotopes of the same element can have different _mass-to-charge ratios.
Implications for Tin’s Properties
The mass-to-charge ratio of an isotope can influence certain physical properties of tin, such as its melting point, boiling point, and density. However, the chemical properties of tin, such as its reactivity, are primarily determined by its atomic number and electron configuration, which are constant across all isotopes.
Understanding the Charge of Tin: A Comprehensive Guide
Embark on a fascinating journey into the realm of tin’s atomic charge, a fundamental aspect that shapes its chemical and physical properties.
Atomic Charge: A Guiding Force
An atom’s charge, attributed to its internal structure, determines its behavior in chemical reactions and interactions. Tin’s atomic charge, a crucial aspect, influences its ability to form bonds, determine reactivity, and contribute to various properties.
Atomic Number and Isotopes: Building Blocks of Tin
The atomic number of tin, a unique identifier, arises from its specific number of protons. Protons, positively charged particles, reside in the atom’s nucleus. Neutrons, their neutral counterparts, also inhabit the nucleus, influencing the atom’s mass.
Tin’s atomic number remains constant, but it exhibits various isotopes. Isotopes are atoms of the same element with varying numbers of neutrons, resulting in differing mass numbers. These variations impact tin’s overall charge distribution.
Electron Configuration: Unveiling Tin’s Quantum World
Electrons, negatively charged particles, occupy specific orbitals around the nucleus. Each orbital is defined by three quantum numbers: principal quantum number, angular momentum quantum number, and magnetic quantum number.
Tin’s electron configuration, the arrangement of its electrons in orbitals, follows periodic trends. Understanding this configuration provides insights into its charge and chemical reactivity.
Valency: The Gateway to Bonding
Valence electrons, residing in the outermost energy level, play a crucial role in chemical bonding. Tin’s valency, determined by the number of valence electrons, governs its ability to form and break bonds with other atoms.
Oxidation States: Redox Reactions Unraveled
When tin participates in chemical reactions, it can undergo changes in its oxidation state, a representation of the number of electrons gained or lost. Oxidation states are crucial for understanding redox reactions, where one species gets oxidized (loses electrons) while another gets reduced (gains electrons).
Ionic Charge: Electrostatic Attraction in Action
Ionic bonding occurs when atoms transfer electrons to achieve a stable electron configuration. The resulting charged ions are held together by electrostatic attraction. Tin can form ions with varying ionic charges, influencing its interactions in ionic compounds.
Applications and Implications: Unlocking Tin’s Potential
Comprehending tin’s charge opens doors to numerous applications and implications in various fields. In chemistry, it guides the design and synthesis of tin-based compounds with specific properties. Metallurgy utilizes this knowledge to control the properties and reactivity of tin alloys. Electronics relies on tin’s properties to create essential components like semiconductors and solder.
Understanding tin’s atomic charge empowers us to harness its versatility, shaping materials and technologies that drive progress in science and innovation.
Diving into the Electron Configuration of Tin: Unveiling Its Periodic Precision
Embark on a captivating journey as we unravel the intricate electron configuration of tin, a fascinating element poised within the enigmatic realm of the periodic table. Unlike mere passengers, electrons occupy specific orbitals around the atom’s nucleus, akin to tiny celestial bodies in an intricate dance. Each electron possesses a unique set of quantum numbers, resembling cosmic blueprints that define their energy levels, shapes, and orientations within the atomic realm.
Delving deeper into tin’s atomic structure, we encounter 50 electrons whirling around a nucleus teeming with 50 positively charged protons. This balance of charges renders tin electrically neutral, yet its electron configuration holds the key to understanding its chemical versatility. Tin’s electrons, like a celestial symphony, occupy seven energy levels, represented by the quantum number n.
As we ascend through these energy levels, we observe an intriguing pattern. The first two energy levels, occupied by two and eight electrons respectively, closely resemble those of noble gases like helium and neon, exuding an aura of stability. It is in the third energy level that tin’s distinctive character emerges, as ten electrons fill the 3s and 3p orbitals.
Navigating further, we reach the fourth energy level, where nature’s artistry truly shines. Here, eight electrons gracefully occupy the 4s and 4p orbitals. This configuration, mirroring the electron configuration of krypton, a noble gas of exceptional stability, bestows upon tin an air of chemical inertness, rendering it less reactive than many of its metallic counterparts.
Recognizing the significance of electron configuration, we can confidently conclude that it orchestrates the periodic trends observed across the elements. Tin, nestled within Group 14 of the periodic table, proudly shares this electron configuration with its groupmates. This kinship manifests in their shared tendency to exhibit a valency of four, contributing to their versatility in forming both covalent and ionic bonds.
Understanding the electron configuration of tin unlocks a treasure trove of insights into its chemical behavior. Like the decipherment of an ancient scroll, these intricate patterns reveal the element’s true nature, empowering us to predict its reactivity, bonding tendencies, and myriad applications in diverse fields, from metallurgy to electronics.
Electron Configuration and the Charge of Tin
As we delve into the realm of quantum mechanics, we encounter orbitals, the mysterious regions where electrons reside. Each orbital possesses three quantum numbers: principal, angular momentum, and spin. These numbers characterize the electron’s energy level, shape, and orientation.
Tin’s electron configuration, 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2, reveals a fascinating pattern. The s and p orbitals have filled subshells, indicating that tin has a stable electron configuration. However, the presence of two electrons in the 5s orbital hints at a potential for charge accumulation.
Charge, as we know, arises from an imbalance between electrons and protons. In a neutral atom like tin, the number of electrons equals the number of protons, resulting in an overall charge of zero. However, if tin gains or loses electrons, this balance is disrupted, leading to the development of a net charge.
The electron configuration of tin suggests that it is more likely to lose electrons than to gain them. This is because the 5s subshell is relatively loosely held, meaning that its electrons can be easily removed. The removal of one or two 5s electrons would give tin a positive charge, making it a cation.
Understanding the electron configuration of tin is crucial for comprehending its charge. It provides insights into the chemical behavior of tin, enabling us to predict its reactions and determine its role in various applications.
The Charge of Tin: Unraveling the Fundamental Nature of the Element
Tin, a lustrous and versatile metal, plays a crucial role in our modern world, from food packaging to electronics. Understanding its charge is essential for comprehending its chemical properties and applications. Let’s embark on a journey to decipher the atomic charge of tin.
Understanding Atomic Charge
At the heart of an atom lies its nucleus, containing protons and neutrons, each carrying a charge. Protons bear a positive charge, while neutrons are neutral. The number of protons defines the atomic number, which is unique for each element and determines its chemical identity. Tin has an atomic number of 50, indicating it has 50 protons in its nucleus.
Valency: The Key to Reactivity
Surrounding the nucleus is an electron cloud, consisting of negatively charged electrons. Electrons occupy specific energy levels called orbitals and are arranged in a fixed order. In particular, the outermost electrons, known as valence electrons, influence the atom’s chemical properties and bonding behavior. Tin possesses four valence electrons, making it quadrivalent.
Valency and the Charge of Tin
Valence electrons determine an atom’s ability to form chemical bonds. When tin forms bonds, it can either donate or accept valence electrons to achieve a stable electron configuration. Depending on the bond type, tin can exhibit a positive charge when it donates electrons or a negative charge when it accepts electrons.
Applications and Implications
Understanding the charge of tin enables us to predict its behavior in various chemical reactions and industrial processes. In metallurgy, its ability to form alloys with other metals is crucial for creating strong and durable materials. In electronics, tin’s properties as a conductor and semiconductor make it indispensable for microchips and other electronic components. Additionally, in chemistry, knowing tin’s charge helps us decipher its reactivity in reactions involving acid-base interactions and redox processes.
Understanding the Valency and Reactivity of Tin
Tin, a versatile metal, plays a crucial role in various applications due to its unique chemical reactivity. This reactivity stems from the valency of tin, a fundamental property that governs its bonding behavior.
Valency refers to the number of electrons in the outermost energy level of an atom, which determine its tendency to form chemical bonds. Tin has four valence electrons, making it a tetravalent element. This means that tin atoms can form up to four covalent bonds with other atoms.
The valency of tin has significant implications for its chemical reactivity. Tin is a moderately reactive metal because it has a high tendency to form covalent bonds. This bonding behavior enables tin to react with a wide range of elements, forming diverse compounds.
For instance, tin can react with halogens (such as chlorine and fluorine) to form tetravalent halides. These halides are ionic compounds, with tin exhibiting a +4 oxidation state. In contrast, when tin reacts with metals (such as sodium and potassium), it forms intermetallic compounds, where tin exhibits a -4 oxidation state.
The valency of tin also affects its catalytic properties. In many industrial processes, tin compounds are used as catalysts to facilitate chemical reactions. The tetravalent nature of tin allows it to form unstable intermediates with reactants, lowering the activation energy of the reaction and making it proceed faster.
By understanding the valency of tin, chemists can predict its chemical reactivity and design materials with specific properties for various applications. This knowledge is essential in fields such as metallurgy, where tin is used to improve the strength and corrosion resistance of alloys, and in electronics, where it is used in soldering and semiconductor fabrication.
Connect valency to the charge of tin.
Understanding the Intriguing Charge of Tin
In the tapestry of chemistry, understanding the charge of an element unlocks the secrets to its behavior and its dance with other elements. Tin, a versatile metal with a rich history, is no exception. Let’s embark on a journey to unravel the enigmatic charge of tin and discover its profound impact on its chemical and physical properties.
Atomic Number and Isotopes: The Building Blocks of Identity
Every atom of tin is defined by its atomic number, the unique number of protons in its nucleus. This number, 50, determines the element’s identity on the periodic table. Along with protons, atoms also contain neutrons, which vary in number, giving rise to different versions of the same element: isotopes. Isotopes of tin have varying mass numbers due to fluctuations in the number of neutrons.
Electron Configuration and Periodic Trends: The Orchestra of Electrons
Electrons, the minuscule particles swirling around the atomic nucleus, play a crucial role in shaping an atom’s charge. Tin‘s electron configuration, a blueprint of its electron distribution, follows the patterns of the periodic table. Its 50 electrons occupy specific orbitals, quantized energy levels arranged in shells. This electron arrangement dictates tin’s position in Group 14 and Period 5, exhibiting a +4 valency.
Valency: The Gateway to Reactivity
Valence electrons, the outermost electrons in an atom’s orbitals, govern its chemical reactivity. Tin, with four valence electrons, has a strong tendency to form stable compounds by sharing or donating these electrons. This valency directly correlates to its charge, as sharing electrons with more electronegative elements results in a partial positive charge for tin, while donating electrons leads to a partial negative charge.
Oxidation States: The Dance of Redox Reactions
Tin exhibits a fascinating range of oxidation states, which represent its capacity to gain or lose electrons in chemical reactions. These states include +2 and +4, with +4 being the most common. Oxidation states are crucial in understanding redox reactions, where elements undergo electron transfer, resulting in changes in their charge. Tin’s ability to change oxidation states makes it a versatile participant in a wide range of chemical transformations.
Understanding the Charge of Tin: A Comprehensive Exploration
Tin, an element with atomic number 50, plays a vital role in various industries and applications. Understanding its charge is crucial for comprehending its chemical and physical properties. This blog post delves into the fascinating world of tin’s charge, exploring its atomic structure, electron configuration, valency, oxidation states, ionic charge, and practical implications.
Atomic Number and Isotopes
Tin’s atomic number defines its identity. It has 50 protons, positively charged particles, in its nucleus. The nucleus also contains neutrons, electrically neutral particles, which influence the mass number of the atom. Tin’s isotopes vary in their neutron count, but all share the same atomic number.
Electron Configuration and Periodic Trends
Electrons, negatively charged particles, orbit the nucleus in specific orbitals, characterized by their energy levels and shapes. Tin’s electron configuration is [Kr]4d¹⁰5s²5p², indicating 50 electrons arranged in various orbitals. This configuration places tin in Group 14 of the periodic table, exhibiting +4 valence electrons.
Valency: Reactivity and Bonding
Valence electrons determine an element’s chemical reactivity. Tin’s four valence electrons make it prone to form covalent bonds with other atoms. This valency contributes to tin’s versatility in forming compounds.
Oxidation States: Redox Reactions
Oxidation states represent the hypothetical charge an atom would have if its electrons were completely lost or gained. Tin exhibits variable oxidation states, commonly +2 and +4. In redox reactions, tin can undergo changes in oxidation states, losing or gaining electrons.
Ionic Charge: Electrostatic Attraction
When tin loses or gains electrons, it becomes an ion, a charged particle. The charge of an ion is determined by the difference between its protons and electrons. Tin can form both positive and negative ions, leading to the formation of ionic compounds.
Applications and Implications
Understanding tin’s charge is essential in diverse fields. In chemistry, it helps predict its reactivity and compound formation. Metallurgy relies on tin’s properties for alloying and corrosion resistance. In electronics, tin’s charge behavior enables its use in semiconductors and solder.
The charge of tin is a multifaceted concept that encompasses its atomic structure, electron configuration, valency, oxidation states, and ionic charge. Grasping these concepts provides a deeper understanding of tin’s chemical and physical properties, enabling its effective utilization in various scientific and technological applications.
The Charge of Tin: A Journey Through the Atom’s Heart
1. Understanding Atomic Charge
Imagine an atom as a miniature universe, teeming with subatomic particles. At its core lies the atomic charge, a fundamental property that governs its chemical and physical behavior. In the case of tin, this charge plays a crucial role in shaping its unique properties.
2. Atomic Number and Isotopes
Within the nucleus, we find protons and neutrons. Protons carry a positive charge, while neutrons remain neutral. The number of protons, known as the atomic number, determines an element’s identity. Tin has an atomic number of 50, meaning it holds 50 protons. Isotopes are atoms of the same element with different numbers of neutrons. While they share the same atomic number, their mass numbers (the sum of protons and neutrons) can vary.
3. Electron Configuration and Periodic Trends
Outside the nucleus, electrons dance in their respective orbitals. The arrangement of electrons, known as electron configuration, adheres to specific rules and periodic trends. Tin has 50 electrons, with its outermost electrons occupying the 5s and 5p orbitals. This configuration places tin in Group 14 of the periodic table, characterized by elements with four valence electrons.
4. Valency: Reactivity and Bonding
Valence electrons are those in the outermost orbitals, and they determine an atom’s chemical reactivity. Tin’s four valence electrons enable it to form stable bonds with other atoms. This property makes tin an important player in a wide range of chemical reactions.
5. Oxidation States: Redox Reactions
Redox reactions involve the transfer of electrons between atoms or ions. Tin can exist in multiple oxidation states, which represent the number of electrons it gains or loses. In these reactions, tin undergoes changes in oxidation state, altering its charge and reactivity. For example, in the reaction Sn + 2HCl → SnCl2 + H2, tin loses two electrons, changing its oxidation state from 0 to +2.
6. Ionic Charge: Electrostatic Attraction
When tin atoms gain or lose electrons, they transform into ions. Ionic bonding occurs when ions with opposite charges attract each other to form stable compounds. The ionic charge of tin depends on the number of electrons it has gained or lost. In the case of SnCl2, tin has lost two electrons, resulting in a ionic charge of +2. This positive charge exerts an electrostatic attraction to the negatively charged chloride ions (-1).
7. Applications and Implications
Understanding the charge of tin is essential in diverse fields. In chemistry, it helps us predict and design chemical reactions. In metallurgy, it influences the properties of tin alloys and their applications in electronics, batteries, and other industries. By unraveling the secrets of atomic charge, we gain insights that drive scientific advancements and shape the world around us.
Explain the oxidation states of tin and its redox properties.
Tin’s Dynamic Charge: Exploring Oxidation States and Redox Reactions
Understanding Atomic Charge
Atomic charge, a fundamental property of elements, governs their chemical and physical behavior. Tin, a versatile metal, showcases unique charge characteristics that shape its properties and applications.
Atomic Number and Isotopes
Tin’s atomic number, 50, reflects the number of protons and electrons in a neutral atom. Isotopes, atoms with varying neutron numbers, alter the mass number while maintaining the same atomic number. Tin has ten stable isotopes, each with a specific neutron-to-proton ratio.
Electron Configuration and Periodic Trends
Tin’s electron configuration, 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²5p², follows the trends of the periodic table. Its outer valence electrons determine its chemical reactivity.
Valency: Reactivity and Bonding
Tin’s four valence electrons grant it a valency of two. This valency governs its tendency to form chemical bonds with other elements, leading to diverse compounds with varying charge characteristics.
Oxidation States: Redox Reactions
Oxidation states represent the formal charge of an atom in a compound. Tin exhibits variable oxidation states, including +2, +4, and +6. These states play a crucial role in redox reactions, chemical transformations involving the transfer of electrons between species. Tin’s ability to change oxidation states makes it a versatile redox agent.
Ionic Charge: Electrostatic Attraction
In ionic bonding, atoms gain or lose electrons to form ions. The resulting electrostatic attraction between ions determines the stability of ionic compounds. Tin can form both positive and negative ions, contributing to its diverse chemical interactions.
Applications and Implications
Understanding tin’s charge characteristics has profound significance in various scientific fields:
- Chemistry: Predicting chemical reactivity and designing novel materials.
- Metallurgy: Tailoring alloys and optimizing metalworking processes.
- Electronics: Developing semiconductors and conducting materials.
The Fascinating Tale of Atomic Charge: Exploring the Secrets of Tin
In the world of elements, tin stands out as a versatile metal with a rich history of applications. Its unique properties stem from its atomic structure and the intricate dance of its electrons, giving it a distinct charge that plays a pivotal role in shaping its behavior.
Understanding Atomic Charge
Every atom carries a charge, which is determined by the number of protons in its nucleus. Protons hold a positive charge, while electrons, found outside the nucleus, carry a negative charge. The overall charge of an atom is the sum of these positive and negative charges. In tin’s case, its atomic number, which represents the number of protons, is 50. This means that each tin atom has a net charge of +50, which is balanced by an equal number of electrons.
The Magic of Isotopes
Tin exists in various forms known as isotopes, which have the same number of protons but different numbers of neutrons. This difference in neutron count alters the mass of the atom but doesn’t affect its charge. Tin has several stable isotopes, the most common of which is ¹²⁰Sn, with 70 neutrons. This isotope accounts for over 96% of all tin atoms in the natural world.
Electron Configuration and Periodic Trends
The arrangement of electrons within an atom’s orbitals, known as its electron configuration, also influences its charge. Tin’s electron configuration, written as [Xe]4f¹⁴5d¹⁰6s², tells us that it has four valence electrons, the electrons that participate in chemical bonding. This configuration places tin in Group 14 of the periodic table, known as the carbon family, where elements share similar chemical properties.
Valency: The Gateway to Chemical Reactivity
Valency refers to the number of electrons that an atom can gain, lose, or share during chemical reactions. Tin’s four valence electrons give it a valency of +4. This means that it has a tendency to form compounds where it shares four electrons with other atoms, achieving a stable electron configuration.
Oxidation States: The Dance of Redox Reactions
Oxidation states are assigned to atoms to represent their charge during chemical reactions. Tin can exhibit multiple oxidation states, including +2 and +4. In an oxidation state of +2, tin has lost two electrons, while in an oxidation state of +4, it has lost four electrons. Oxidation states play a crucial role in understanding the reactivity of tin in redox reactions, where electrons are transferred between atoms.
Ionic Charge: The Power of Electrostatic Attraction
Ionic bonding occurs when atoms transfer electrons to achieve a stable electron configuration. Tin can form positive ions, known as cations, by losing electrons. The number of electrons lost determines the ionic charge. For example, a tin cation with a charge of +2 has lost two electrons, giving it a net charge of +2. These positively charged ions can electrostatically attract negatively charged ions, forming ionic compounds.
Understanding the Charge of Tin: A Comprehensive Guide
In the realm of chemistry, understanding the atomic charge of elements is crucial for unraveling their properties and behavior. Tin, a versatile and widely used metal, holds a fascinating story about its charge, and we’re diving into the depths to reveal its secrets.
Atomic Number and Isotopes
The atomic number of an element defines its identity in the periodic table. Tin boasts an atomic number of 50, meaning it has 50 protons within its nucleus. Complementing the protons are neutrons, which contribute to the element’s mass but have no electrical charge. Together, protons and neutrons determine the atomic mass of tin.
Isotopes are variations of the same element, sharing the same atomic number but differing in the number of neutrons. Tin has ten stable isotopes, with mass numbers ranging from 112 to 124. These isotopes have varying neutron counts, affecting their masses but maintaining their chemical properties.
Electron Configuration and Periodic Trends
Moving beyond the nucleus, we encounter electrons, the negative counterparts to protons. Their arrangement within energy levels, known as orbitals, defines the element’s electron configuration.
Tin has an electron configuration of [Kr] 4d¹⁰ 5s² 5p². This configuration places it within Group 14 of the periodic table and aligns with its chemical properties. Notably, tin has four valence electrons, which play a crucial role in bonding.
Valency: Reactivity and Bonding
Valence electrons are the outermost electrons, determining an element’s chemical reactivity. Tin’s four valence electrons allow it to form covalent bonds by sharing electrons with other atoms. This valency is directly related to the charge of tin in compounds.
Oxidation States: Redox Reactions
Oxidation states represent the charge an atom would have if its electrons were completely transferred. Tin exhibits multiple oxidation states, including +2 and +4. In redox reactions, tin can undergo changes in oxidation states, gaining or losing electrons to form compounds.
Electrostatic Attraction and Ionic Charge
In the realm of ionic bonding, atoms transfer electrons to achieve stable electron configurations. The resulting ions have a net electrical charge. Electrostatic attraction plays a significant role in determining the ionic charge of tin.
When tin loses two valence electrons, it becomes a cation with a charge of +2. This positive charge attracts negatively charged ions, forming ionic compounds. Conversely, when tin gains four valence electrons, it becomes an anion with a charge of -4.
Applications and Implications
Comprehending the charge of tin opens doors to countless applications. In chemistry, it aids in predicting chemical reactivity and bonding behavior. In metallurgy, it guides the development of alloys and other materials. In electronics, it paves the way for efficient and reliable devices.
Understanding the charge of tin is a testament to the intricate tapestry of chemical properties that define elements. By unraveling these secrets, we gain a deeper appreciation for the building blocks of our world and their impact on our technological advancements.
Unveiling the Charge of Tin: A Comprehensive Guide to Its Atomic and Ionic Nature
Tin, a versatile and widely used metal, plays a pivotal role in numerous industries and applications. Understanding its atomic and ionic charge is crucial for comprehending its chemical behavior and properties. This article will delve into the various aspects of tin’s charge, providing a comprehensive overview of its atomic charge, electron configuration, valency, oxidation states, and ionic charge.
Atomic Charge: The Foundation of Chemical Understanding
The atomic charge of an element refers to the net electrical charge of its atom. In the case of tin, its atomic charge is determined by the number of protons (positively charged particles) and electrons (negatively charged particles) present in its nucleus and electron cloud, respectively. A neutral atom has an atomic charge of zero, indicating that the number of protons equals the number of electrons.
Isotopes and Atomic Number
Isotopes are atoms of the same element with varying numbers of neutrons. While the number of protons defines an element’s atomic number, the number of neutrons can vary, resulting in different isotopes. For instance, tin has several stable isotopes, including tin-112, tin-116, and tin-120. These isotopes have the same atomic number (50) but differ in their number of neutrons, influencing their mass but not their atomic charge.
Electron Configuration: Shaping Chemical Properties
The electron configuration of an element describes the arrangement of its electrons within specific energy levels or orbitals. Tin’s electron configuration is represented as [Kr] 4d¹⁰ 5s² 5p², indicating that it has two electrons in the outermost energy level, known as valence electrons. These valence electrons largely determine the chemical reactivity and bonding behavior of tin.
Valency: The Gateway to Chemical Bonding
Valency refers to the combining capacity of an element, determined by the number of valence electrons it possesses. In the case of tin, it has four valence electrons, making it tetravalent. Tin can form chemical bonds by sharing or transferring these valence electrons with other atoms, creating compounds with varying oxidation states.
Oxidation States: Unveiling Redox Chemistry
Oxidation states assign a specific charge to an atom within a compound, indicating its degree of oxidation or reduction. Tin can exhibit various oxidation states, including +2 and +4, commonly found in its compounds. Redox reactions involve the transfer of electrons between atoms, changing their oxidation states.
Ionic Charge: Shaping Electrostatic Interactions
Ionic charge refers to the electrical charge carried by an atom when it loses or gains electrons, resulting in the formation of ions. Tin can form positively charged ions (Sn²⁺ or Sn⁴⁺) by losing valence electrons. In ionic compounds, these positively charged tin ions are attracted to negatively charged ions, forming stable crystal structures due to electrostatic attraction.
Applications and Implications
Understanding the charge of tin has significant implications in various fields:
- Chemistry: It aids in predicting the reactivity, bonding behavior, and redox properties of tin-containing compounds.
- Metallurgy: Knowledge of ionic charge is essential for designing alloys and controlling corrosion processes.
- Electronics: Tin-based compounds are used in semiconductors, solder, and conductive materials, where its charge plays a crucial role in determining their electrical properties.
By comprehending the atomic charge, electron configuration, valency, oxidation states, and ionic charge of tin, we gain a deeper insight into its behavior and unlock its potential applications in numerous scientific and technological domains.
Unveiling the Secrets of Tin’s Charge: A Journey into Its Atomic Identity
Prologue:
In the vast tapestry of elements, tin stands out with its unique properties and multifaceted nature. Its charge, a fundamental aspect of its atomic identity, holds the key to understanding its behavior and interactions in the world of chemistry. Embark on this captivating journey as we unravel the secrets of tin’s atomic charge.
Atomic Charge: The Foundation
Atomic charge refers to the overall electrical charge carried by an atom, which is determined by the difference between the number of positively charged protons and negatively charged electrons. In the case of tin, its atomic charge is crucial for understanding its chemical and physical characteristics.
Unveiling the Atomic Structure
Protons and neutrons, the building blocks of the atomic nucleus, define tin’s atomic number. Isotopes, different forms of an element with varying neutron counts, provide insights into the variations in tin’s mass number. Exploring these fundamental particles paves the way for a deeper understanding of tin’s atomic charge.
Electron Configuration: A Quantum Dance
Orbitals, regions where electrons reside, and quantum numbers, describing electron energy levels, shape the electron configuration of tin. Delving into these concepts reveals how tin’s electrons contribute to its overall charge.
Valency: The Key to Reactivity
Valence electrons, the outermost electrons in an atom, play a pivotal role in chemical bonding and reactivity. Tin’s valency, influenced by its electron configuration, governs its ability to form bonds and interact with other elements.
Oxidation States: Unveiling Redox Transformations
Oxidation states represent the charge an atom appears to have when involved in chemical reactions. As tin gains or loses electrons during redox reactions, its oxidation state changes, shedding light on its redox properties and versatility in bonding.
Ionic Charge: Electrostatic Attractions
When tin forms ionic bonds, it gains or loses electrons, resulting in the formation of ions with a net charge. Understanding electrostatic attraction and its influence on ionic charge reveals the stability and reactivity of tin in ionic compounds.
Applications and Implications
Comprehending tin’s charge is not merely an academic pursuit but holds profound implications in various fields. From the development of alloys in metallurgy to the fabrication of semiconductors in electronics, understanding tin’s charge unlocks its potential and enables its use in countless applications.
Epilogue:
The charge of tin, a seemingly complex concept, becomes a fascinating narrative when explored through the lens of atomic structure, electron behavior, and chemical bonding. By unraveling its secrets, we gain a deeper appreciation for this versatile element and its contributions to our technological advancements and scientific discoveries.
The Intriguing Charge of Tin: A Comprehensive Guide
Journey into the Realm of Atomic Chemistry
From the depths of the periodic table emerges a captivating element named tin. Its unique charge plays a pivotal role in determining its behavior and unravels a fascinating tale of atomic structure and chemical interactions. Let’s embark on a journey to explore the enigmatic charge of tin.
Delving into Atomic Charge
Atomic charge, the net electrical charge of an atom, is a fundamental property that drives the chemistry of tin. Understanding this charge is crucial for unraveling its properties and unlocking its potential in various fields.
Unraveling Atomic Structure
Protons, the atomic workhorses, reside in the nucleus, bestowing upon tin its atomic number, which is unique to each element. Neutrons, their neutral counterparts, add to the atom’s mass number. Together, these particles define the atom’s identity.
Electron Configuration: The Blueprint of Reactivity
Electrons occupy specific energy levels around the nucleus, each with its own orbital shape and quantum numbers. Tin’s electron configuration reveals its valence electrons, the gatekeepers of reactivity. These electrons determine tin’s ability to bond and form compounds.
Valency: The Key to Chemical Bonding
Valency, the number of valence electrons, dictates tin’s role in chemical reactions. Its four valence electrons equip tin with the ability to form a wide range of bonds.
Oxidation States: Redox Reactions Revealed
In redox reactions, atoms undergo changes in their oxidation states, which reflect the number of electrons gained or lost. Tin exhibits several oxidation states, including +4 and +2, showcasing its versatile redox behavior.
Ionic Charge: Electrostatic Attraction at Play
Ionic bonding arises when atoms lose or gain electrons, resulting in the formation of charged particles called ions. Electrostatic attraction between oppositely charged ions creates ionic compounds. Tin’s ionic charge is crucial for its stability and reactivity in these compounds.
Applications: Unlocking Tin’s Potential
Harnessing the understanding of tin’s charge unlocks a wealth of applications:
- Metallurgy: Tin’s resistance to corrosion makes it an essential component in alloys used for soldering and food packaging.
- Chemistry: Tin compounds find use as catalysts in various chemical reactions, enhancing their efficiency and selectivity.
- Electronics: Tin’s conductivity and solderability make it a crucial material in printed circuit boards and electronic components.
Understanding the charge of tin grants us a profound insight into its chemical and physical properties. From atomic structure to chemical reactivity, the charge of tin shapes its behavior and opens up a world of possibilities across multiple disciplines. As we delve deeper into the world of tin, its enigmatic charge continues to fascinate and inspire.