Unlocking Triple Bonds: A Comprehensive Guide To High-Energy Covalent Connections
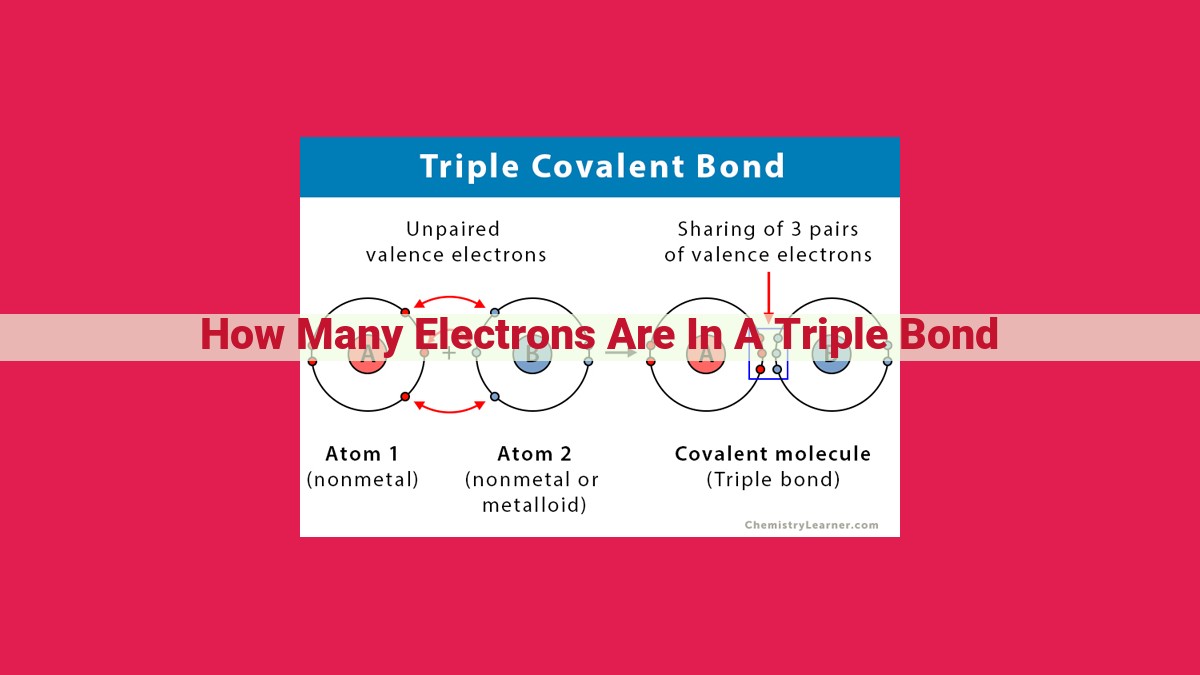
Triple bonds are high-energy covalent bonds formed by the sharing of six electrons between two atoms. In Lewis dot structures, triple bonds are represented by three parallel lines between the bonded atoms. To form a triple bond, each atom contributes three valence electrons, resulting in a total of six shared electrons. Triple bonds are stronger than double or single bonds due to their increased number of shared electrons and shorter bond lengths. They are commonly found in molecules such as acetylene (C2H2) and carbon dioxide (CO2).
Unveiling the Strength and Structure of Triple Bonds
In the captivating world of molecules, the presence of triple bonds stands out as a testament to the remarkable forces that govern their architecture. These chemical marvels, formed by the covalent bond between three pairs of electrons, play a pivotal role in determining the shape, reactivity, and stability of countless organic compounds.
Triple bonds possess a unique characteristic that sets them apart from their single and double bond counterparts: they consist of one sigma bond and two pi bonds. The sigma bond is formed by the head-on overlap of atomic orbitals, while the pi bonds are created by the lateral overlap of perpendicular orbitals. This intricate bonding arrangement results in an incredibly strong and rigid molecular connection.
Moreover, triple bonds exhibit shorter bond lengths and higher bond energies compared to their single and double bond counterparts. This increased bond strength and rigidity translate into a greater stability and resistance to molecular deformation.
The formation of triple bonds is a fascinating process that requires a careful balance of valence electrons, the electrons that participate in chemical bonding. Atoms that readily form triple bonds are typically those with four valence electrons, such as nitrogen and carbon.
As these atoms interact, their valence electrons undergo a series of hybridization and promotions to form three equivalent atomic orbitals that can overlap and create the triple bond. This complex process results in the formation of a highly stable and energetically favorable molecular structure.
Understanding triple bonds is essential for unraveling the intricate tapestry of molecular interactions. Their unique properties have profound implications in fields ranging from organic chemistry and materials science to enzyme catalysis and pharmaceutical research. By comprehending the nature and significance of triple bonds, we gain deeper insights into the fundamental principles that govern the chemical world.
Lewis Dot Structures and Valence Electrons
When we delve into the intricate world of molecules, we encounter the concept of valence electrons. These are the electrons that reside in the outermost shell of an atom, eager to participate in the bonding dance that creates molecules. To visualize this electron ballet, chemists employ a handy tool called a Lewis dot structure.
In a Lewis dot structure, each atom is represented by its atomic symbol, surrounded by a constellation of dots representing its valence electrons. Triple bonds, as their name suggests, are formed when three pairs of valence electrons are shared between two atoms. This intimate sharing creates a solid line connecting the atoms in a Lewis dot structure.
For instance, let’s consider the molecule acetylene (C₂H₂). Each carbon atom has four valence electrons, while each hydrogen atom has one. To form a triple bond, the carbon atoms contribute three pairs of valence electrons each, while the hydrogen atoms contribute their single electrons. This results in a triple bond between the carbons, represented in its Lewis dot structure as:
**H:C:::C:H**
Formation of Triple Bonds
In the realm of molecular structures, triple bonds represent a fascinating and intriguing concept. To unravel the secrets of triple bond formation, let us delve into the role of valence electrons and the captivating idea of covalent bonding.
The Ballroom of Bonding
Imagine valence electrons as dancers, each eager to find a partner to dance the waltz of chemical bonding. In the case of triple bonds, three pairs of electrons decide to join forces, creating a harmonious connection between two atoms. They share their delicate hands, forming an intimate dance that we call a covalent bond.
A Triple Swan Dive
The triple bond is akin to a swan dive performed in perfect unison. Each electron pair contributes its energy, creating a bond that is stronger than the sum of its parts. This synchronized movement results in a shorter bond length and a higher bond energy, making the triple bond an exceptional force in the molecular world.
The Butterfly Effect of Valence Electrons
The existence of triple bonds depends on the availability of valence electrons. Atoms with a scarcity of valence electrons are unlikely to form triple bonds, preferring instead to engage in less demanding bonding arrangements. Conversely, atoms with an abundance of valence electrons become eager triple bond enthusiasts, ready to share their riches with eager partners.
A Molecular Tango
Covalent bonding, the driving force behind triple bond formation, is a graceful tango between atoms. The electrons, our talented dancers, move between the nuclei of bonded atoms, creating a shared space where positive and negative charges intertwine harmoniously.
Bond Strength and Properties of Triple Bonds
Triple bonds are the strongest type of covalent bond, consisting of three shared electron pairs between two atoms. They are more powerful than double and single bonds due to the higher number of shared electrons. This increased electron density results in a shorter bond length and higher bond energy.
Triple bonds typically have bond lengths of around 1.2-1.3 angstroms, significantly shorter than double and single bonds. This is because the triple bond’s three shared electron pairs create a stronger attraction between the atoms, drawing them closer together. The high bond energy of triple bonds indicates the amount of energy required to break them, making them highly stable and less reactive.
Additionally, triple bonds possess directional rigidity, meaning they can only form in a straight line between the bonded atoms. They cannot rotate or bend like single or double bonds, contributing to the stability and strength of the molecule.
Examples of molecules containing triple bonds include acetylene (C2H2), hydrogen cyanide (HCN), and carbon dioxide (CO2). Acetylene, in particular, is a highly reactive gas used in welding and cutting due to the strong bond between its two carbon atoms.
Understanding Triple Bonds: Delving into Their Formation and Nature
Welcome to the captivating world of triple bonds, a realm of molecular chemistry that showcases the intricate interplay of electrons and their pivotal role in shaping the structure and properties of matter. In this exploration, we’ll embark on a journey to unravel the mysteries of triple bonds, examining their formation, strength, and the underlying principles that govern their behavior.
Molecular Orbital Theory: A Quantum Perspective
At the heart of understanding triple bonds lies molecular orbital theory, a sophisticated framework that transcends the limitations of Lewis dot structures. This theory employs the concept of molecular orbitals, which are hypothetical 3D regions where electrons reside. In the case of triple bonds, a combination of three molecular orbitals comes into play, forming a central strong sigma bond and two weaker pi bonds.
Sigma (σ) bond: This head-on overlap of orbitals occurs along the internuclear axis, resembling a dumbbell shape. It forms the foundation of the strong covalent bond that characterizes triple bonds.
Pi (π) bonds: In contrast to sigma bonds, pi bonds are formed by the lateral overlap of p orbitals. They are perpendicular to the σ bond, creating a double-bond-like structure that further strengthens the bond system.
Triple Bond Formation: A Tale of Valence Electrons
The formation of triple bonds hinges on the availability of sufficient valence electrons. These electrons, residing in the outermost energy level of an atom, seek to achieve a stable electron configuration of eight electrons, known as the octet rule.
When two atoms approach each other with six valence electrons each, they can form a triple bond by sharing all six electrons. This shared electron pool forms the three molecular orbitals that constitute the triple bond. The sharing of a triple bond results in an incredibly strong bond compared to single or double bonds.
Bond Strength and Properties: A Testament to Triple Bond Eminence
Triple bonds boast exceptional strength and stability, outshining their single and double bond counterparts. Their bond lengths are shorter, and their bond energies are higher, reflecting the robust nature of the triple bond system.
Molecules containing triple bonds exhibit unique characteristics that stem from their rigid molecular geometry. This rigidity can influence chemical reactivity, stability, and physical properties, making triple bonds important players in various chemical processes.
Applications of Triple Bonds: Beyond Theory and Into the Real World
Triple bonds are not mere theoretical constructs; they play essential roles in a vast array of chemical compounds and biological systems. From the rigid backbone of DNA to the fuel-efficient combustion of alkynes, triple bonds contribute to the structure, function, and behavior of countless molecules.
In conclusion, triple bonds are captivating chemical entities born from the interplay of valence electrons and the principles of molecular orbital theory. Their remarkable strength, unique properties, and widespread applications make them vital players in the chemical world. By exploring the intricate nature of triple bonds, we gain a deeper appreciation for the fundamental forces that shape our molecular universe.