Unlocking The Inaccessible: Exploring The Inert Nature Of Free Nitrogen
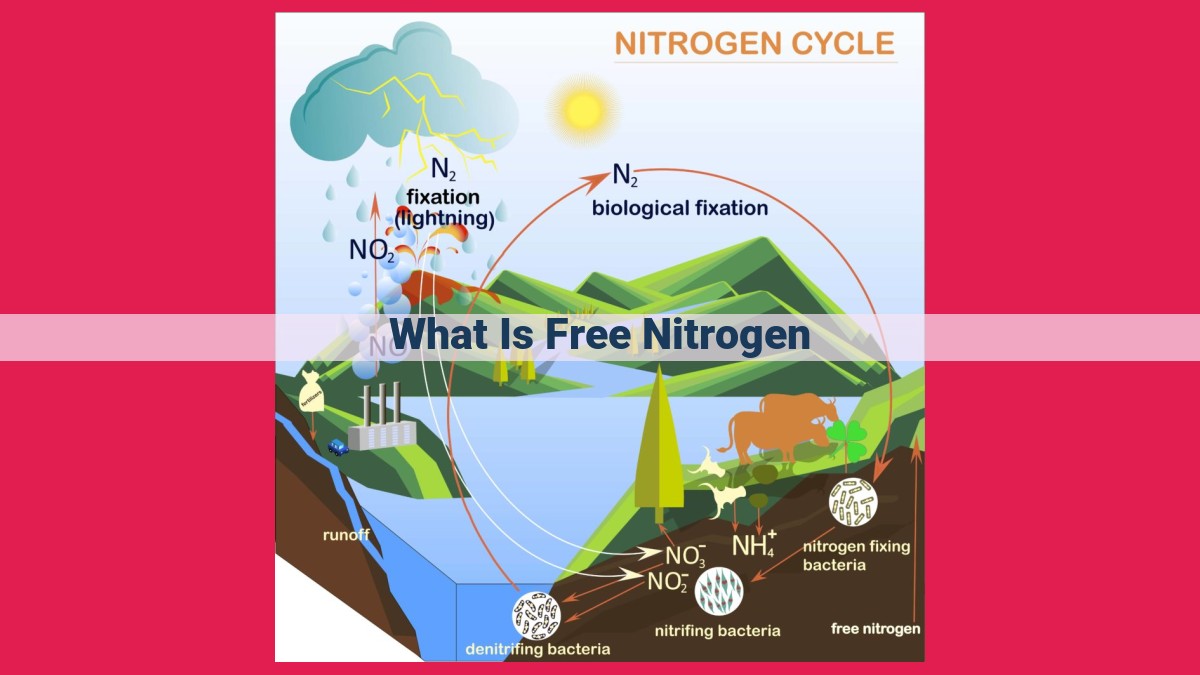
Free nitrogen refers to the inert form of nitrogen gas (N2) that makes up approximately 78% of Earth’s atmosphere. Despite its abundance, free nitrogen is largely inaccessible to most organisms due to its triple-bonded structure, which makes it resistant to reactions and renders it unavailable for biological use.
Nitrogen: Unveiling its Reactive and Inert Nature
Nitrogen, a paradoxical element, showcases dual characteristics, revealing its versatility in the intricate tapestry of our planet. In its inert form, it dominates the Earth’s atmosphere, composing nearly 80% of its mass. Yet, within the nitrogen cycle, it sheds its reactive nature, undergoing transformations that sustain life.
Inert Atmospheric Nitrogen
Atmospheric nitrogen, a massive reservoir, remains largely unreactive due to its exceptionally stable triple bond. This inertness ensures the stability of our atmosphere and provides a protective shield against harmful solar radiation. Nitrogen-fixing bacteria and industrial processes harness the energy to break this bond, unlocking nitrogen’s hidden potential.
Reactive Nitrogen Cycle
In the nitrogen cycle, nitrogen undergoes a series of transformations, from inert atmospheric nitrogen to usable forms for biological processes. Specialized bacteria, such as Rhizobium and Frankia, possess the remarkable ability to fix nitrogen, converting it into ammonia. This crucial step makes nitrogen accessible to plants and microorganisms.
The nitrogen cycle relentlessly cycles through a series of reactions, ensuring the continuous supply of usable nitrogen. Plants assimilate nitrogen from the soil and use it to synthesize essential compounds like proteins and nucleic acids. Animals consume plants, transferring nitrogen through the food chain to sustain their own biological processes. Eventually, decomposers return nitrogen to the soil, completing the cycle’s intricate dance.
Nitrogen Fixation: The Alchemy of Life
In the symphony of life, nitrogen plays a pivotal role, yet its transformation from an inert gas to a vital nutrient is a tale of remarkable ingenuity. This process, known as nitrogen fixation, is the alchemy that unlocks the door to life on Earth.
Biological Nitrogen Fixation: The Soil’s Magicians
Nature’s master chemists, bacteria, possess the ability to harness the power of enzymes to break the triple bond in atmospheric nitrogen, transforming it into ammonia – a form that plants can readily absorb. This biological nitrogen fixation is the backbone of the nitrogen cycle, ensuring a continuous supply of this essential nutrient in the ecosystem.
Industrial Nitrogen Fixation: The Haber-Bosch Process
Human ingenuity mirrored nature’s magic with the development of the Haber-Bosch process, an industrial tour de force that synthesizes ammonia from atmospheric nitrogen and hydrogen. This breakthrough made fertilizers widely available, enabling a green revolution that boosted crop yields and fed a burgeoning global population.
The Significance: Unleashing Nitrogen’s Potential
Nitrogen fixation is pivotal for life’s flourishing. Fixed nitrogen becomes the building block of proteins, nucleic acids, and other essential biomolecules. It supports plant growth, fostering healthy ecosystems and feeding all living creatures. Without nitrogen fixation, the web of life would swiftly unravel.
Nitrogen’s Journey: Unveiling the Movement of Life’s Essential Element
Nitrogen, the quintessential element of life, embarks on an extraordinary journey through the environment, weaving its way through various compartments, each with a vital role to play. Its adventures begin in the vast expanse of the atmosphere, where it exists as inert _N2, accounting for approximately 78% of our planet’s air.
The Nitrogen Cycle: Nature’s Masterpiece
From the celestial heights, nitrogen descends to Earth, embarking on a transformative odyssey known as the nitrogen cycle. This intricate process involves a series of natural and industrial mechanisms that convert inert N2 into usable forms for biological processes.
Atmospheric Nitrogen: The Source and Sink
Atmospheric nitrogen serves as the primary reservoir of N2. Its transformation begins with natural events such as lightning strikes, which release NO2 and NO into the atmosphere. These compounds react with water and oxygen to form HNO3 (nitric acid), which falls to the ground as acid rain, replenishing soil nitrogen.
Soil Nitrogen: The Microbial Orchestra
Soil nitrogen undergoes a fascinating metamorphosis, thanks to the maestro microorganisms that reside there. Bacteria, both aerobic and anaerobic, engage in a symphony of transformations, converting N2 into ammonia (_NH3_)**, _nitrites (_NO2_)**, and _nitrates (_NO3_)**. These _nitrogenous compounds are essential nutrients for plants, eagerly absorbed through their roots.
Plant Nitrogen: The Foundation of Life
Nitrogen plays an indispensable role in plant physiology. It is the backbone of chlorophyll, the green pigment that enables photosynthesis, the process by which plants convert sunlight into energy. Nitrogen is also a key component of proteins, the building blocks of life. As plants synthesize proteins, they absorb nitrogen from the soil, incorporating it into their tissues.
Animal Nitrogen: The Nutrient Cascade
Nitrogen continues its journey through the food chain as animals consume plants. Herbivores, like cows and sheep, ingest nitrogen in the form of plant proteins, while carnivores, like lions and tigers, obtain nitrogen from consuming herbivores. Through this cascade of predation, nitrogen is transferred from plants to animals, supporting animal nutrition and growth.
Nitrogen’s journey through the environment is a continuous cycle, an unfolding narrative of transformation and interdependence. It is a testament to the remarkable resilience of nature and the profound significance of this essential element for life on Earth.