Unlocking Molarity Secrets: A Comprehensive Guide To Titration For Accurate Solution Concentration Determination
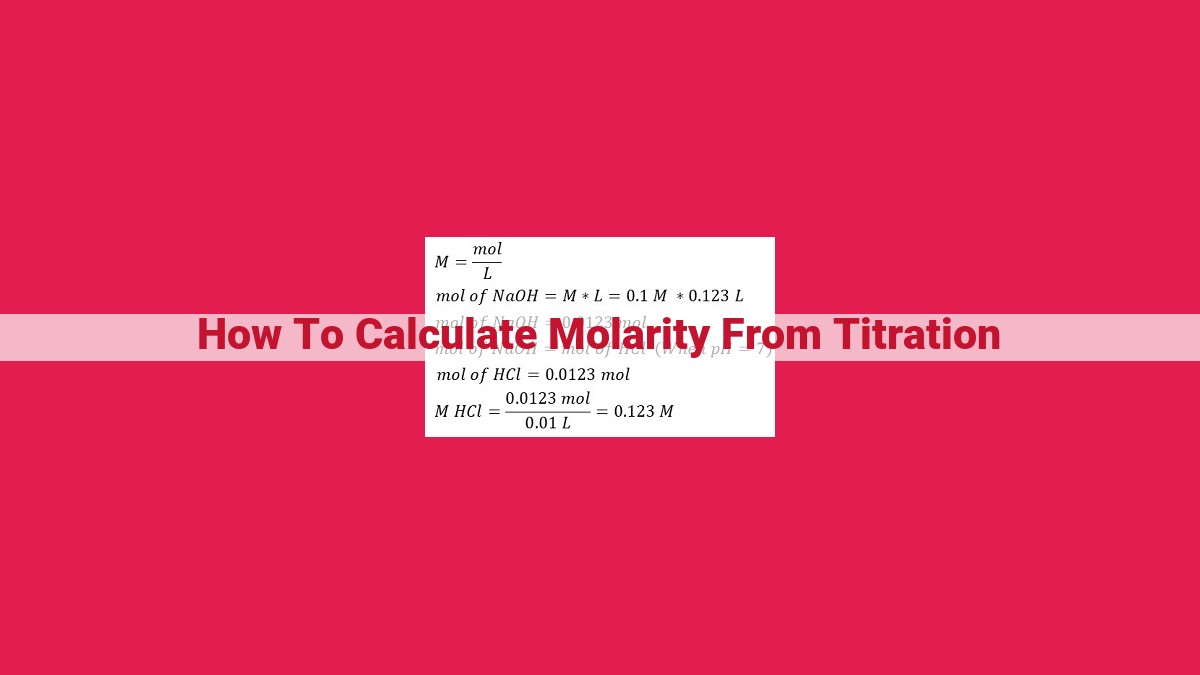
Titration, a fundamental technique in chemistry, involves determining the concentration of a solution by reacting it with a solution of known concentration. Molarity (M) is a measure of concentration, defined as the moles of solute per liter of solution. To calculate molarity from titration, several key concepts are used: moles, equivalence point, and balanced chemical equation. By understanding these concepts, scientists can accurately determine molarity, a crucial factor in chemical calculations and applications in analytical chemistry and biochemistry.
Titration, a cornerstone of chemistry, plays a crucial role in determining the unknown concentration (molarity) of a solution by precisely measuring the volume of another solution of known concentration required to react completely. This quantitative analysis technique involves a stepwise addition of the known solution (titrant) to the unknown solution (analyte) until the equivalence point is reached.
At the equivalence point, the moles of the analyte and titrant are equal, and the chemical reaction between them is complete. This point is critically important because it allows us to calculate the molarity of the unknown solution using the known molarity of the titrant.
Molarity (M) is a fundamental concept in chemistry, representing the number of moles of a solute per liter of solution. It’s a key parameter in stoichiometry, enabling us to determine the quantitative relationship between reactants and products in a chemical reaction. By understanding molarity, we can accurately predict the amount of reactants and products involved in a chemical process.
Fundamental Concepts: The Building Blocks of Molarity
In the fascinating world of chemistry, titration plays a pivotal role in unraveling the unknown. This technique, like a culinary adventure, involves the careful addition of a known molarity solution (analogous to seasoning) to a solution of unknown concentration (the mystery ingredient). Through this process, we can deduce the exact moles of reactants involved, a crucial piece of the chemical puzzle.
Molarity, symbolized as M, is a measure of a solution’s concentration. It signifies the number of moles of a substance dissolved in exactly one liter of solution. Understanding molarity allows us to quantify the exact amount of reactants and products in a reaction, akin to having a precise recipe in the kitchen.
Equivalence point, a pivotal concept in titration, marks the moment when the moles of reactant and moles of added solution are perfectly balanced. Identifying this point is essential for determining molarity.
A balanced chemical equation serves as the blueprint for chemical reactions, indicating the exact proportions of reactants and products involved. It provides a roadmap for calculating molarity and predicting the outcome of a reaction.
Finally, stoichiometry, the language of chemistry, helps us decode the ratios of reactants and products involved in a balanced chemical equation. It’s like translating the blueprint into understandable instructions, allowing us to calculate equivalence point, moles, and ultimately molarity.
Understanding these fundamental concepts is akin to mastering the art of chemistry; they equip us with the tools to comprehend chemical reactions and predict their outcomes. These concepts are the foundation upon which the calculation of molarity rests, a vital step in unraveling the mysteries of the molecular world.
Calculating Molarity from Titration: A Step-by-Step Guide
Understanding the Importance of Accuracy and Precision
In titration, accuracy and precision are crucial for reliable molarity calculations. Accurate measurements ensure that the data you collect truly reflects the actual values, while precision means that repeated measurements produce consistent results. This accuracy and precision will translate into a more precise calculation of molarity.
Step 1: Gather the Necessary Data
Before you can calculate molarity, you’ll need to collect the following data from your titration:
- The volume of the titrant (the solution with known concentration) used in the titration
- The molarity of the titrant
- The balanced chemical equation for the reaction between the titrant and analyte (the solution of unknown concentration)
Step 2: Calculate the Moles of Titrant Used
Using the volume and molarity of the titrant, you can calculate the moles of titrant used in the reaction:
Moles of Titrant = Volume of Titrant (in liters) x Molarity of Titrant (in moles/liter)
Step 3: Determine the Equivalence Point
The equivalence point is the point in the titration where the moles of titrant added are exactly equal to the moles of analyte present. This is where the reaction is complete. Identifying the equivalence point is crucial for determining the molarity of the analyte.
Step 4: Use Stoichiometry to Find Moles of Analyte
Based on the balanced chemical equation, you can determine the mole ratio between the titrant and analyte. Using this ratio, you can calculate the moles of analyte present:
Moles of Analyte = Moles of Titrant x (Mole Ratio of Analyte/Mole Ratio of Titrant)
Step 5: Calculate the Molarity of the Analyte
Finally, using the moles of analyte and the volume of the analyte solution, you can calculate the molarity of the analyte:
Molarity of Analyte = Moles of Analyte / Volume of Analyte Solution (in liters)
Example Calculation
Note: In a real experiment, the values will differ.
- Volume of titrant used: 25.00 mL (0.025 L)
- Molarity of titrant: 0.100 M
- Balanced chemical equation: NaOH + HCl → NaCl + H2O (1:1 mole ratio)
Step 1: Calculate moles of titrant used
Moles of Titrant = 0.025 L x 0.100 M = 0.0025 mol
Step 2: Determine the equivalence point (not applicable in this example)
Step 3: Calculate moles of analyte
Moles of Analyte = 0.0025 mol x 1 = 0.0025 mol
Step 4: Calculate the molarity of the analyte
Molarity of Analyte = 0.0025 mol / 0.050 L = 0.050 M
Therefore, the molarity of the analyte is 0.050 M.
Applications of Molarity: A Cornerstone in Chemical Calculations
Molarity, a fundamental concept in chemistry, transcends its role in titration. It finds widespread applications in diverse fields, opening doors to a deeper understanding of chemical processes. Let’s delve into the captivating world of molarity’s applications.
Analytical Chemistry: Unveiling the Mysteries of Chemical Composition
In the realm of analytical chemistry, molarity shines as a beacon of precision. It empowers chemists to meticulously quantify the concentration of substances in complex mixtures. Titration, a cornerstone of analytical chemistry, relies heavily on molarity to determine the exact amount of a known substance present in a solution. By carefully measuring the volume of a titrant with known molarity, analysts can calculate the concentration of the unknown substance through stoichiometric calculations.
Biochemistry: Deciphering the Language of Life
Molarity plays a pivotal role in biochemistry, the meeting point of chemistry and biology. It provides a common language for understanding the intricate workings of biological molecules. For instance, scientists measure the concentration of enzymes, the workhorses of cellular reactions, in terms of molarity. This information helps researchers determine enzyme activity and unravel the complex web of biochemical pathways.
Pharmaceutical Industry: Ensuring Precision in Drug Development
The pharmaceutical industry relies heavily on molarity to ensure the potency of life-saving medications. Before a drug can be administered to patients, its concentration must be precisely controlled. Molarity enables pharmacists and pharmaceutical companies to formulate drugs with the exact concentration required for therapeutic efficacy.
Environmental Science: Guardians of Our Planet
In the field of environmental science, molarity is a vital tool for monitoring water quality. By measuring the molarity of pollutants in water samples, scientists can assess the extent of contamination and develop mitigation strategies. Similarly, molarity plays a crucial role in studying the concentration of nutrients in soil, ensuring optimal crop growth.
In summary, molarity is a versatile and indispensable concept in chemistry. Its applications span a vast array of fields, from analytical chemistry to biochemistry, the pharmaceutical industry, and environmental science. Understanding molarity empowers chemists, biologists, pharmacists, and environmental scientists to unravel the mysteries of chemical composition, decipher the language of life, ensure the potency of medications, and protect our planet. Embrace the power of molarity in your pursuit of chemical knowledge, and unlock a world of discoveries that await your exploration.