Temperature’s Impact: Unlocking Diffusion Rates
Temperature profoundly affects the rate of diffusion by influencing the kinetic energy of molecules. As temperature rises, the kinetic energy of molecules increases, leading to more frequent and energetic collisions between molecules. These increased collisions facilitate the movement of molecules across a concentration gradient, resulting in a faster rate of diffusion. This temperature dependence is quantified by the diffusion coefficient, which increases with increasing temperature, indicating a stronger driving force for diffusion.
- Explain what diffusion is and how it works.
Diffusion: The Invisible Dance of Molecules
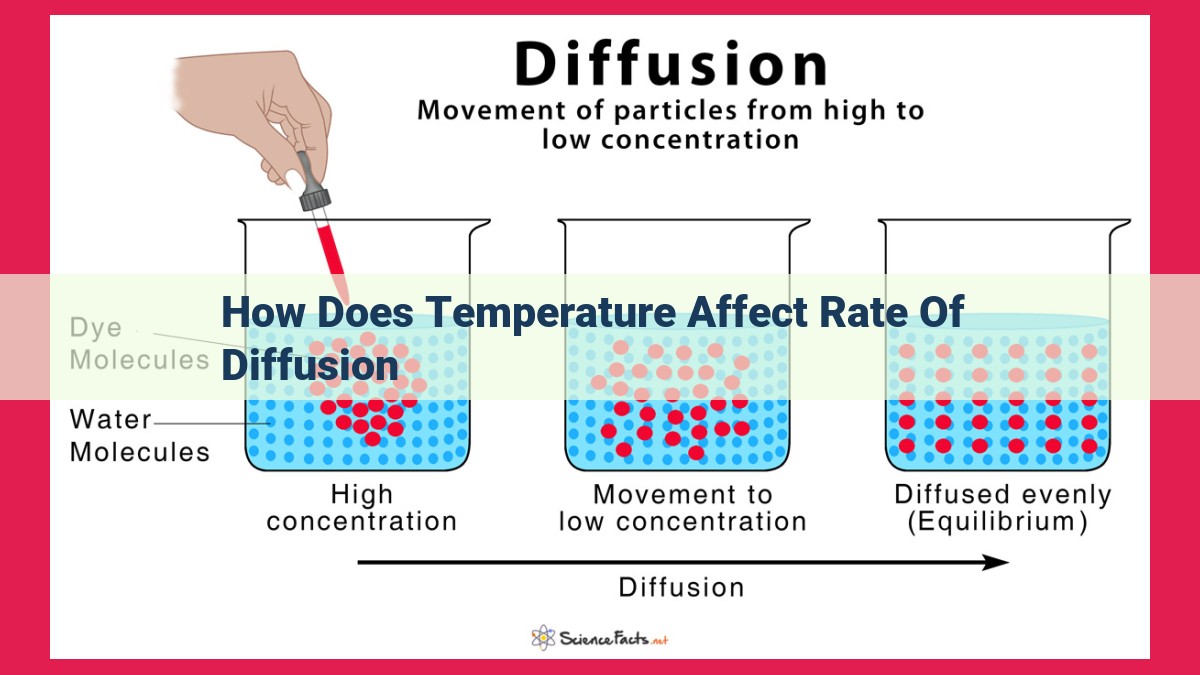
In the realm of science, there exists a fascinating phenomenon known as diffusion, a process that drives the movement of molecules from areas of high concentration to areas of low concentration. It’s an invisible dance that occurs constantly around us, shaping the world in myriad ways.
How Diffusion Works
Imagine a room filled with perfume. When the bottle is opened, the perfume molecules disperse throughout the space. This happens because the molecules have kinetic energy, causing them to move and collide with each other. As they collide, they transfer their energy, spreading the perfume molecules evenly throughout the room. This process is diffusion.
The concentration gradient is the difference in concentration between two regions. It serves as the “driving force” behind diffusion, as molecules naturally flow from areas of high concentration to areas of low concentration, seeking equilibrium.
Rate of Diffusion: Unraveling the Influential Factors
In the world of science, diffusion plays a crucial role in various processes, from the transport of nutrients in living organisms to the spreading of gases in the atmosphere. Understanding the factors that influence the rate of diffusion is essential to comprehend these processes effectively.
Temperature: The Catalyst for Molecular Motion
Temperature stands out as a primary factor that governs the rate of diffusion. As temperature increases, the kinetic energy of molecules also rises. This amplified energy fuels the increased motion of molecules, leading to more frequent collisions between them.
Molecular Size: A Limiting Factor
The size of molecules also impacts the rate of diffusion. Larger molecules encounter more obstacles in their path, thereby slowing down their diffusion. Conversely, smaller molecules can maneuver more easily, resulting in a faster diffusion rate.
Concentration Gradient: Driving the Movement
The concentration gradient, a measure of the difference in the concentration of a substance between two points, acts as a driving force for diffusion. A steeper concentration gradient creates a stronger driving force, causing molecules to diffuse more rapidly from areas of higher concentration to areas of lower concentration.
Diffusion Path: Obstacles and Facilitators
The physical characteristics of the diffusion path can also influence the rate of diffusion. Barriers, such as membranes or gels, can impede the movement of molecules, slowing down the diffusion process. Conversely, facilitators, such as channels or pores, can enhance diffusion by providing pathways for molecules to traverse more efficiently.
Viscosity: Resistance to Molecular Movement
Viscosity measures the resistance of a fluid to flow. Higher viscosity creates greater resistance to molecular movement, lowering the rate of diffusion. This is particularly relevant in biological systems, where fluids like blood and cytoplasm can significantly impact diffusion.
Understanding the Rate of Diffusion
By considering these factors, we gain insights into the dynamics of diffusion and its role in various scientific processes. From the movement of nutrients in living organisms to the exchange of gases in the atmosphere, the rate of diffusion shapes the behavior of molecules and influences the outcomes of countless physical and biological phenomena.
Temperature: The Driving Force Behind Kinetic Energy
In the realm of diffusion, temperature plays a crucial role in orchestrating the movement of molecules. Picture a bustling dance floor teeming with dancers representing molecules. Temperature is like the DJ, influencing the dancers’ energy levels.
As the temperature rises, the kinetic energy of the molecules increases. This translates to more vigorous dance moves, resulting in a frenzy of collisions between molecules. The increased frequency and intensity of these collisions create the driving force behind diffusion.
Imagine the dance floor as a crowded space where molecules struggle to navigate. With low kinetic energy, the dancers move sluggishly, bumping into each other less often, leading to a slow rate of diffusion. However, when the temperature rises, the dancers become more energetic, their movements more frantic. This surge in collisions expedites the rate of diffusion, allowing molecules to spread more quickly and efficiently.
Kinetic Energy: Fueling Increased Collisions
In the realm of diffusion, kinetic energy plays a pivotal role. It acts as the driving force behind collisions between molecules, ultimately influencing the rate of diffusion.
Picture this: molecules are in a constant state of motion, whizzing around like tiny cars on a racecourse. The higher the temperature, the faster these molecules move, as their kinetic energy increases. Think of it like hot air balloons on a windy day – the hotter the air, the more they soar and bounce off each other.
As molecules gain momentum, they collide more frequently. These collisions are like tiny bumper cars, bouncing off each other and changing direction. It’s these collisions that allow molecules to spread out and facilitate diffusion.
With every collision, molecules gain a new trajectory, increasing the likelihood of them moving in the direction of a concentration gradient. Imagine a crowded room filled with people trying to get to the exit. The more people collide and shuffle, the faster they’ll spread out and fill the room.
Therefore, increased kinetic energy directly leads to more collisions, which in turn propels the diffusion process. The faster the molecules move and collide, the quicker they’ll spread out and reach their destination.
Increased Collisions: The Foundation for Faster Diffusion
Diffusion, the process by which molecules spread out from an area of high concentration to low concentration, is driven by the random motion of molecules. The rate of diffusion depends on several factors, and one of the most important is temperature.
Temperature and kinetic energy are closely related. As temperature increases, so does the average kinetic energy of molecules. This is because increased temperature means molecules vibrate faster and with more energy. The higher the kinetic energy, the more collisions occur between molecules.
Collisions between molecules are an important part of diffusion because they allow molecules to move from areas of high concentration to low concentration. The more collisions that occur, the faster the rate of diffusion. This is because the increased collisions create more opportunities for molecules to move in the direction of the concentration gradient.
The rate of diffusion is also affected by the diffusion coefficient, which is a measure of how easily molecules can move through a medium. The diffusion coefficient depends on the size of the molecules, the temperature, and the viscosity of the medium.
Diffusion Coefficient: Quantifying Diffusion
- Define the diffusion coefficient and explain its significance.
Diffusion Coefficient: Quantifying Diffusion
Imagine yourself in a crowded room filled with people. As you walk through the crowd, you’ll notice that you have to squeeze and dodge to get by. The more crowded the room, the harder it is to move. Just like our movement in a crowd, the movement of molecules in a fluid is also hindered by the presence of other molecules.
This hindrance to molecular movement is known as diffusion. Diffusion is the process by which molecules spread out and mix evenly throughout a space. The rate at which diffusion occurs depends on several factors, including temperature.
When the temperature increases, the kinetic energy of the molecules also increases. Kinetic energy is the energy of motion. As the kinetic energy increases, the molecules move faster and collide more frequently with each other. These increased collisions lead to a faster rate of diffusion.
To quantify diffusion, scientists use a parameter called the diffusion coefficient. The diffusion coefficient is a measure of how quickly molecules diffuse through a given medium. It depends on the size and shape of the molecules, as well as the temperature of the medium.
At higher temperatures, the diffusion coefficient increases because the molecules have higher kinetic energy and collide more frequently. Therefore, the relationship between temperature and the diffusion coefficient is directly proportional.
Understanding the diffusion coefficient is crucial in various fields, including biology, chemistry, and engineering. It helps researchers predict the rate of diffusion of drugs, nutrients, and other substances in cells, tissues, and materials. By manipulating temperature and other factors that influence the diffusion coefficient, scientists can control the rate of diffusion and optimize processes in a wide range of applications.
Temperature Dependence of Diffusion Coefficient: Unraveling the Interplay
In the realm of diffusion, where molecules embark on a continuous journey, temperature reigns as a potent maestro, orchestrating the pace of this molecular dance. Understanding the intricate relationship between temperature and the diffusion coefficient is crucial for comprehending the dynamics of molecular movement.
The diffusion coefficient serves as a quantifier of diffusion, measuring the rate at which molecules spread out within a medium. It’s a pivotal parameter that underlies various biological and industrial processes, ranging from drug delivery to the spread of pollutants.
Now, let’s delve into the fascinating tapestry of temperature’s influence on the diffusion coefficient. As temperature rises, it imparts a surge of kinetic energy to the molecules, the microscopic fuel that drives their incessant collisions. With increased kinetic energy, molecules traverse greater distances and experience more frequent encounters, which in turn leads to a higher diffusion coefficient.
This dependence of the diffusion coefficient on temperature is manifested in various natural and industrial scenarios. For instance, in an aqueous environment, the diffusion coefficient of molecules increases by approximately 1-2% for every degree Celsius of temperature rise. This fundamental relationship finds applications in diverse fields, such as the design of drug delivery systems and the modeling of environmental processes.
Comprehending the temperature dependence of the diffusion coefficient empowers scientists and researchers to manipulate diffusion rates, optimize processes, and predict the behavior of molecules in a multitude of contexts. By harnessing this knowledge, we can unlock the potential of diffusion in countless applications, from enhancing medical treatments to safeguarding our environment.