Unlocking Chemical Processes: Exploring Potential Energy Surfaces For Reaction Mechanisms
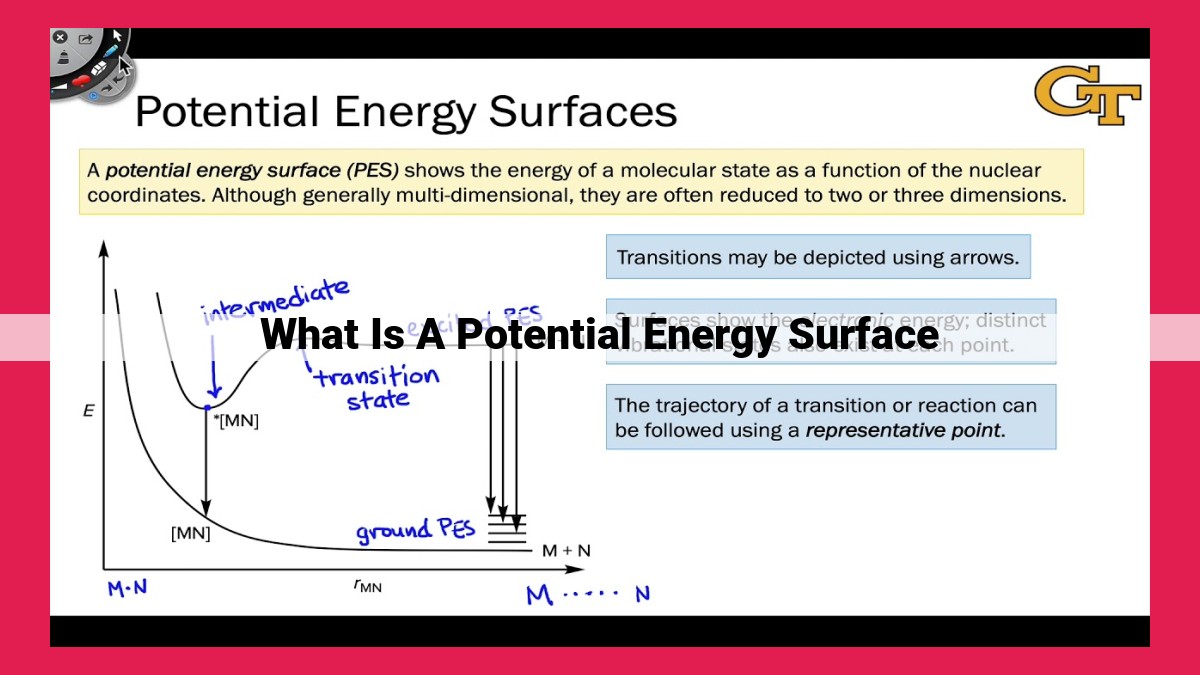
A potential energy surface (PES) describes the energetic landscape of a multi-particle system, mapping the potential energy as a function of atomic coordinates. It provides insights into reaction mechanisms by identifying the pathways, energy barriers, and transition states involved in chemical transformations. The PES is a powerful tool in chemistry, enabling predictions of reaction courses, understanding bonding and reactivity, and facilitating computational simulations of chemical processes.
Delving into the Enigma of Potential Energy Surfaces: The Landscape of Chemical Reactions
Imagine a dynamic symphony of atoms and molecules, each with its unique energy profile, dancing within a multi-dimensional potential energy surface. This surface, a captivating landscape of energy, is the key to understanding the intricate choreography of chemical reactions.
In essence, the potential energy surface is a blueprint that chronicles the energy changes as atoms rearrange and molecules transform. It reveals the hidden paths that reactants take to become products, the energy barriers they must overcome, and the secrets of chemical bonding and reactivity.
Consider a system of two atoms, such as hydrogen and oxygen. As they approach, their potential energy fluctuates, creating a surface with peaks and valleys. The lowest point represents the molecular bond, while the highest point signifies the transition state, the critical juncture where the old bonds break and new ones form.
Delving into the Foundations of Potential Energy Surfaces
In the realm of multi-particle systems, the concept of a potential energy surface emerges as a powerful tool for understanding the intricate dance of atomic and molecular interactions. Imagine a landscape of undulating hills and valleys, where each point represents the energy state of the system as its particles move and interact. This intricate landscape is known as the potential energy surface.
At the heart of this concept lies the Born-Oppenheimer Approximation, a simplification that allows us to separate the movement of electrons from the heavier nuclei. As electrons move much faster than nuclei, we can effectively freeze the nuclei in place and focus on the electronic energy alone. This approximation greatly simplifies the calculation of potential energy surfaces.
Navigating this energy landscape, we encounter the concept of the reaction coordinate, a pathway that connects the initial reactants to the final products. Tracing this path reveals the changing energy profile of the system as it undergoes transformation. At the peak of this energy barrier lies the transition state, the pinnacle of the reaction, representing the cusp of change.
Related to the transition state is the saddle point, a pivotal location where the gradient of the potential energy surface is zero. This point corresponds to the transition state, offering a glimpse into the delicate balance as the system teeters on the brink of transformation.
Beyond the transition state, the intrinsic reaction coordinate emerges as a path of least resistance, guiding the system towards reactants or products. This path represents the lowest energy pathway, revealing the preferred route for chemical transformations.
Finally, the minimum energy path completes our understanding of the potential energy surface. This path connects reactants and products along a series of lowest energy states, providing insights into the most favorable pathway for chemical reactions.
The Significance of Potential Energy Surfaces: A Powerful Tool in Chemistry
The potential energy surface (PES) is a fundamental concept in chemistry that plays a crucial role in understanding and predicting chemical processes. It’s a multi-dimensional landscape that maps out the energy changes that occur as atoms and molecules interact.
Predicting Reaction Course
PES enables chemists to visualize the energy landscape of a chemical reaction. It identifies the reactant and product states, as well as the transition state, the highest energy point along the reaction pathway. This information is invaluable for understanding the reaction mechanism, predicting reaction rates, and identifying potential catalysts.
Understanding Bonding and Reactivity
PES helps us understand the forces that govern chemical bonding and reactivity. By examining the shape and contours of the PES, chemists can determine the stability of molecules, the strength of bonds, and the likelihood of chemical reactions. This knowledge is essential for designing new materials with desired properties and understanding the behavior of molecules in complex systems.
Computational Applications
PES is a powerful tool for computational methods in chemistry. It allows chemists to simulate and analyze chemical processes at the atomic level. By solving the Schrödinger equation on the PES, researchers can calculate reaction rates, predict product distributions, and explore the behavior of complex molecular systems. This has revolutionized the field of computational chemistry and led to significant advancements in drug design, materials science, and many other areas.
In summary, the potential energy surface is a fundamental concept that underpins our understanding of chemical reactions, bonding, and reactivity. By providing a roadmap of the energy landscape, PES empowers chemists to predict reaction outcomes, design new molecules, and simulate chemical processes at the atomic level. It’s a powerful tool that has transformed the field of chemistry and continues to play a vital role in advancing our knowledge of the molecular world.