Unlocking The Secrets Of Carbon: Its Atomic Structure And Chemical Significance
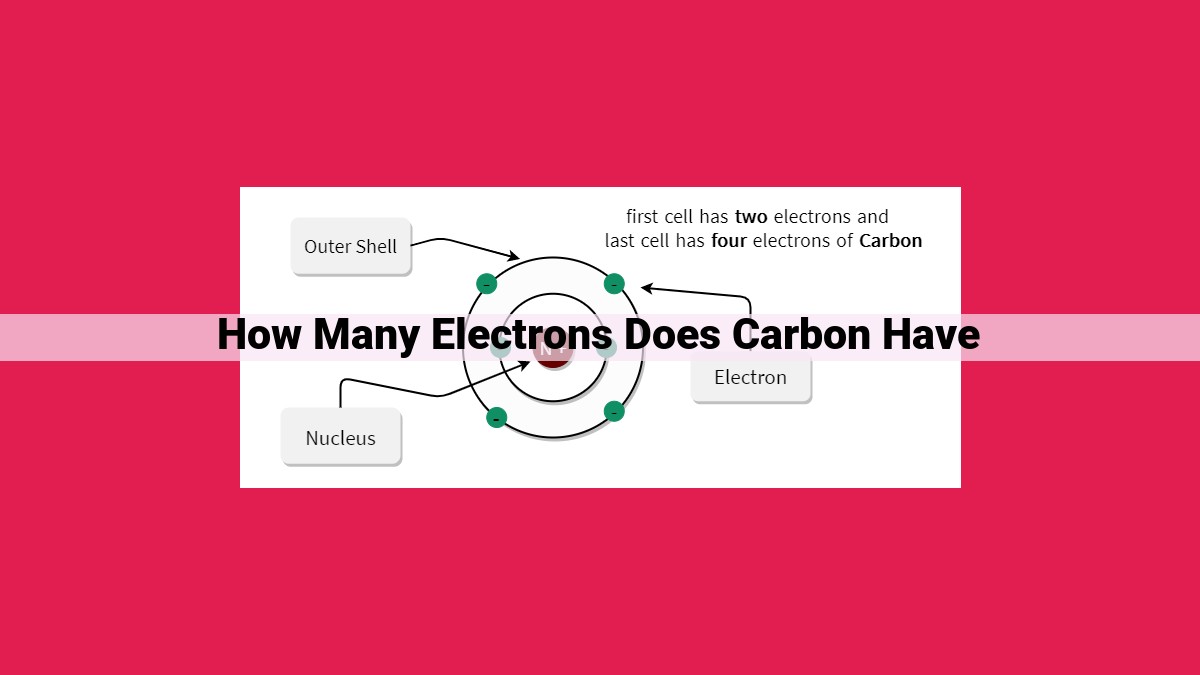
Carbon, an essential element in organic and inorganic chemistry, possesses a unique atomic structure that determines its behavior. With an atomic number of 6, carbon has 6 electrons, arranged in two energy levels: two in the first level and four in the second. These electrons play a crucial role in carbon’s chemical bonding, enabling it to form a diverse range of compounds with other elements.
Electrons and Carbon: A Story of Atoms and Chemistry
Have you ever wondered why carbon is such an extraordinary element? It’s the building block of life; it forms the backbone of our DNA and the very molecules that make us who we are.
But what makes carbon so special? It’s all in its electrons. In this blog post, we’ll embark on a journey to uncover the secrets of carbon’s atomic structure, electron configuration, and how these fundamental properties shape its unique characteristics.
Atomic Structure: The Basics
Imagine an atom as a tiny universe, with protons and neutrons forming the dense nucleus at its core. These particles determine the element’s identity. Orbiting around the nucleus are electrons, like planets around a star. Electrons are the key to understanding an element’s chemical behavior.
Carbon’s Atomic Structure
Carbon has six protons, giving it an atomic number of 6. This means that every carbon atom has six positively charged protons in its nucleus. Balancing out these protons are six negatively charged electrons, which dance around the nucleus in specific orbits.
Electron Configuration: A Blueprint for Bonding
Electrons aren’t randomly distributed; they follow a specific arrangement called electron configuration. Think of it as a blueprint for how electrons are organized within an atom’s energy levels.
Carbon has two energy levels. The first level is filled with two electrons, while the second level has four electrons. These four valence electrons are the ones responsible for carbon’s interactions with other atoms, forming the chemical bonds that create the diverse molecules we know.
Atomic Structure of Carbon: Unraveling the Building Blocks of Life
Prepare to embark on an atomic adventure as we unravel the fundamental composition of carbon, the element that forms the backbone of all living organisms. Let’s dive deep into the heart of a carbon atom, uncovering its secrets one subatomic particle at a time.
The Basic Building Blocks of an Atom:
Imagine an atom as a miniature solar system, with a tiny, dense nucleus as its sun. The nucleus contains two types of subatomic particles: positively charged protons and neutral neutrons. Orbiting the nucleus like celestial bodies are negatively charged electrons.
Carbon’s Atomic Number: A Unique Identity
Within the vast universe of elements, each one has a unique atomic number. This number represents the number of protons in the nucleus. Carbon’s atomic number is 6, signifying that every carbon atom possesses 6 protons. This distinctive characteristic defines carbon’s position on the periodic table and governs its chemical properties.
Electron Configuration of Carbon: Unveiling the Building Blocks of Life
In the tapestry of chemistry, understanding the electron configuration of elements is akin to deciphering the fundamental language of matter. Carbon, the cornerstone of all living organisms and the versatile element that shapes our world, holds a unique place in this chemical lexicon.
Electron Configuration: A Map of Energy Levels
Picture an atom as a miniature solar system, with its nucleus as the sun and electrons orbiting it like planets. The arrangement of electrons in these orbits, known as energy levels, defines an element’s electron configuration. Each energy level can accommodate a specific number of electrons, and they are filled in a sequential order.
Carbon’s Energy Levels and Electron Arrangement
Carbon’s atomic number, which represents the number of protons in its nucleus, is 6. This also determines the number of electrons it possesses. The first two electrons occupy the innermost energy level, while the remaining four reside in the second level. The electron configuration of carbon can be expressed as:
1s² 2s² 2p²
where “s” and “p” designate the shapes of the electron orbitals.
Valence Electrons: The Key to Reactivity
The electrons in the outermost energy level, the second level in carbon’s case, are known as valence electrons. These electrons play a crucial role in determining the chemical behavior of an element. Carbon’s four valence electrons make it a versatile bonding partner, enabling it to form a wide range of compounds with other elements.
Implications for Chemistry: A Language of Bonding
Understanding electron configuration is essential in unraveling the intricacies of chemistry. It helps us predict the bonding behavior of elements based on their valence electrons. Carbon’s four valence electrons allow it to form covalent bonds with other atoms, leading to the formation of countless organic molecules that underpin life and technology.
The electron configuration of carbon is a blueprint that unlocks the secrets of its chemical nature. It reveals the element’s ability to bond and form the complex structures that make life possible. Understanding electron configuration is a fundamental step towards comprehending the language of chemistry and appreciating the wonders of our carbon-based world.
Calculating the Number of Electrons in a Carbon Atom
The intriguing world of chemistry often revolves around investigating the mysterious building blocks of matter—atoms. Among these atoms, carbon stands out as a star player, showcasing a remarkable ability to form diverse compounds that shape our existence.
Unraveling the number of electrons in a carbon atom is a pivotal step towards understanding its unique properties. To embark on this journey, we must first delve into the fascinating realm of atomic structure.
Atomic Structure of Carbon: A Microscopic Journey
Imagine an atom as a miniature solar system, with a nucleus at its core and electrons orbiting around it. Protons, positively charged, reside within the nucleus, while neutrons remain electrically neutral. Electrons, the negatively charged entities, dance in energy levels like tiny planets.
Carbon, with an atomic number of 6, harbors an equal number of protons in its nucleus, endowing it with a distinctive identity. This atomic number signifies that carbon’s nucleus also contains 6 neutrons, resulting in an atomic mass of 12.
Electron Configuration: Unveiling the Electron Arrangement
Electrons occupy specific energy levels, each characterized by its energy and shape. Carbon’s electrons are distributed in two energy levels: 1s and 2s. The 1s level holds two electrons, while the 2s level accommodates four.
This intricate arrangement, known as electron configuration, plays a crucial role in determining carbon’s chemical behavior. The outermost electrons, termed valence electrons, engage in chemical interactions, creating bonds with other atoms. In carbon’s case, it possesses four valence electrons.
Chemical Bonding: Carbon’s Versatile Connections
Carbon’s versatility in forming bonds stems from its valence electrons. These electrons eagerly participate in covalent bonding, sharing electrons with other atoms. Covalent bonds form the foundation of organic molecules, the backbone of life on Earth.
Carbon can form single, double, or triple bonds, depending on the number of shared electrons. In methane, for example, carbon forms four single bonds with four hydrogen atoms. In ethylene, carbon engages in a double bond with another carbon atom, and in acetylene, it forms a triple bond.
The number of electrons in a carbon atom, 6, holds profound implications for its chemical behavior. Carbon’s four valence electrons allow it to form diverse covalent bonds, giving rise to the myriad of organic molecules that shape our world.
Comprehending electron configurations is essential in chemistry, as they dictate an element’s chemical properties and reactivity. Carbon’s electron configuration, with its unique number of valence electrons, sets the stage for its remarkable ability to bond and create the intricate tapestry of life.
The Significance of Electron Configuration in Chemistry
In the vast realm of chemistry, understanding electron configurations holds immense significance. It serves as a fundamental concept upon which our comprehension of chemical properties and reactivity rests.
Electron configuration refers to the specific arrangement of electrons within an atom’s energy levels. These energy levels are quantized, meaning they can only exist at certain fixed values. Each energy level can accommodate a limited number of electrons, and the outermost energy level plays a crucial role in determining an element’s chemical behavior.
The electron configuration of an element provides vital information about its:
-
Chemical bonding: Elements interact with each other by forming chemical bonds. The electron configuration dictates the number of valence electrons (electrons in the outermost energy level) an element has, which directly influences its bonding abilities. Elements with similar valence electron configurations exhibit similar chemical bonding patterns.
-
Chemical properties: The electron configuration also influences an element’s chemical properties, such as its reactivity, electronegativity, and ionization energy. Elements with similar electron configurations tend to have similar chemical properties. By understanding electron configurations, chemists can predict the behavior of elements in chemical reactions.
-
Reactivity: The ease with which an element undergoes chemical reactions depends on its electron configuration. Elements with low ionization energies (easily lose electrons) or high electron affinities (easily gain electrons) are generally more reactive. Electron configuration provides insights into the tendency of elements to lose or gain electrons, thus predicting their reactivity.
Understanding electron configurations is paramount for comprehending the behavior of elements and their interactions with each other. It forms the bedrock of chemistry, enabling scientists to decipher the intricate dance of chemical reactions and predict the properties of new materials.