Unlocking Atomic Identity: The Essential Role Of Atomic Number In Tin’s Characterization
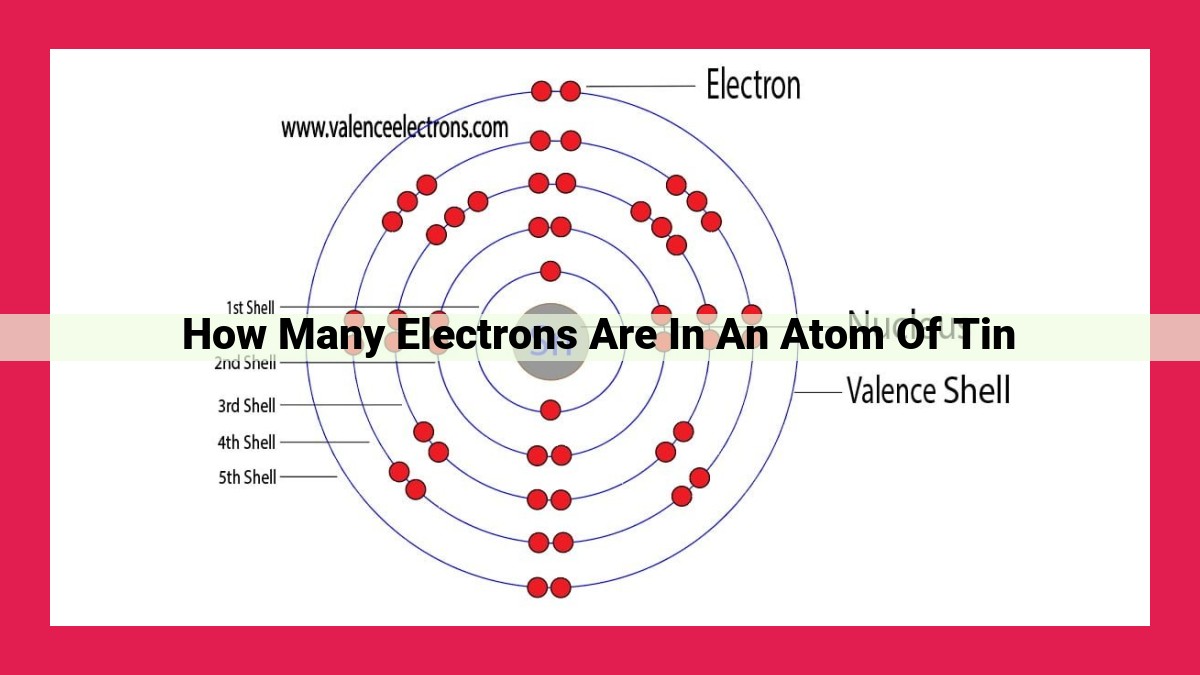
The atomic number of tin (Sn) is 50, indicating the number of protons and electrons in a neutral atom.
Atomic Number: The Ultimate Identity Card for Atoms
Atomic Number: The Ultimate Identity Card for Atoms
In the vast expanse of the universe, atoms are the fundamental building blocks of everything that exists. Each atom possesses a unique identity card that sets it apart from all others – its atomic number. This number, embedded deep within the heart of an atom, is a telltale sign of its elemental nature.
Imagine an element as a family, and the atomic number as each family member’s unique birthdate. Just as we use our birthdates to identify ourselves, the atomic number identifies the element to which an atom belongs. For example, all atoms with an atomic number of 1 are hydrogen, while those with an atomic number of 6 are carbon.
The atomic number doesn’t only reveal an atom’s elemental identity; it also governs its electrical properties. This number determines the number of protons within the atom’s nucleus. Protons, with their positive charge, attract negatively charged electrons, forming the stable structures we recognize as atoms. The electrical attraction between protons and electrons is the driving force behind chemical reactions, allowing atoms to bond and form molecules, the building blocks of our world.
Electron Configuration: Unraveling the Blueprint of Atoms
Imagine the atom as a miniature solar system, with electrons orbiting their nucleus like planets. To fully comprehend the behavior and properties of atoms, understanding their electron configuration is paramount.
Orbitals: The Electron’s Celestial Dance Floor
Electrons occupy specific regions around the nucleus called orbitals. These orbitals are like cosmic dance halls, where electrons move in predetermined patterns. Just as each planet has its own orbit, each electron occupies a unique orbital with a specific energy level.
Valence Electrons: The Social Butterflies of Atoms
Not all orbitals are created equal. The outermost orbitals, known as valence orbitals, play a crucial role in determining an atom’s chemical reactivity. Valence electrons are the electrons that participate in chemical bonds, forming the basis of interactions between atoms.
Quantum Numbers: Decoding the Electron’s Identity
Every electron is characterized by a unique set of four quantum numbers. These numbers provide essential information about the electron’s energy, shape, orientation, and spin. By decoding these quantum numbers, scientists can discern the intricate details of an electron’s existence within an atom.
Electron Shell: Energy Levels in the Atomic Cosmos
Every atom is a miniature universe, with electrons orbiting the nucleus in a harmonious dance. These electrons reside in specific energy levels, known as electron shells, which play a crucial role in defining an atom’s behavior and properties.
Imagine the nucleus as the sun, and the electron shells as concentric rings of planets orbiting around it. Each shell can hold a specific number of electrons, with the first shell closest to the nucleus holding the fewest. As we move outward, each shell holds more electrons, creating a layered structure.
The energy levels within each shell vary, with electrons occupying orbitals, which are like individual “houses” within these energy levels. The closer an electron is to the nucleus, the lower its energy level. Electrons in higher energy levels are more loosely bound to the nucleus, making them more reactive and likely to participate in chemical reactions.
The way electrons are distributed across the shells and orbitals is determined by quantum numbers, which describe the specific properties of each electron. Each electron has three quantum numbers: principal, angular momentum, and magnetic. These numbers uniquely identify an electron’s state within an atom, similar to an address that pinpoints its location.
Understanding electron shells is essential for comprehending the behavior of atoms. It allows us to predict how atoms will interact with each other, forming molecules and shaping the world around us. From the smallest chemical reactions to the vastness of the universe, the electron shell is a fundamental building block that helps us unravel the mysteries of our atomic world.
Noble Gas Configuration: The Golden Standard of Stability
Noble Gas Configuration: The Golden Standard of Stability
In the realm of atoms, stability is the ultimate goal. Like the peaceful citizens of a harmonious society, atoms strive to achieve a state of equilibrium, where their electronic structure grants them immunity from change.
Inert Gases: The Guardians of Stability
Noble gases stand as the epitome of atomic tranquility. With their outer electron shells filled to capacity, these elements are content and uninterested in participating in chemical reactions. They possess an inert nature, showcasing the stability that comes with a fully occupied electron configuration.
The Allure of Stability
Atoms, like humans, have an innate longing for stability. They seek to imitate the noble gases, arranging their electrons in a way that minimizes their energy and maximizes their contentment. This quest for stability drives a fundamental force in chemistry, as atoms strive to shed or gain electrons to achieve the coveted noble gas configuration.
In conclusion, noble gas configuration represents the pinnacle of atomic stability. Atoms of all kinds strive to emulate the peaceful existence of noble gases, ensuring a harmonious balance within the microscopic world. By understanding the importance of this golden standard, we gain a deeper appreciation for the complex and fascinating nature of our atomic surroundings.