Units Of Energy: A Comprehensive Guide For Engineering, Physics, Biology, And Nutrition
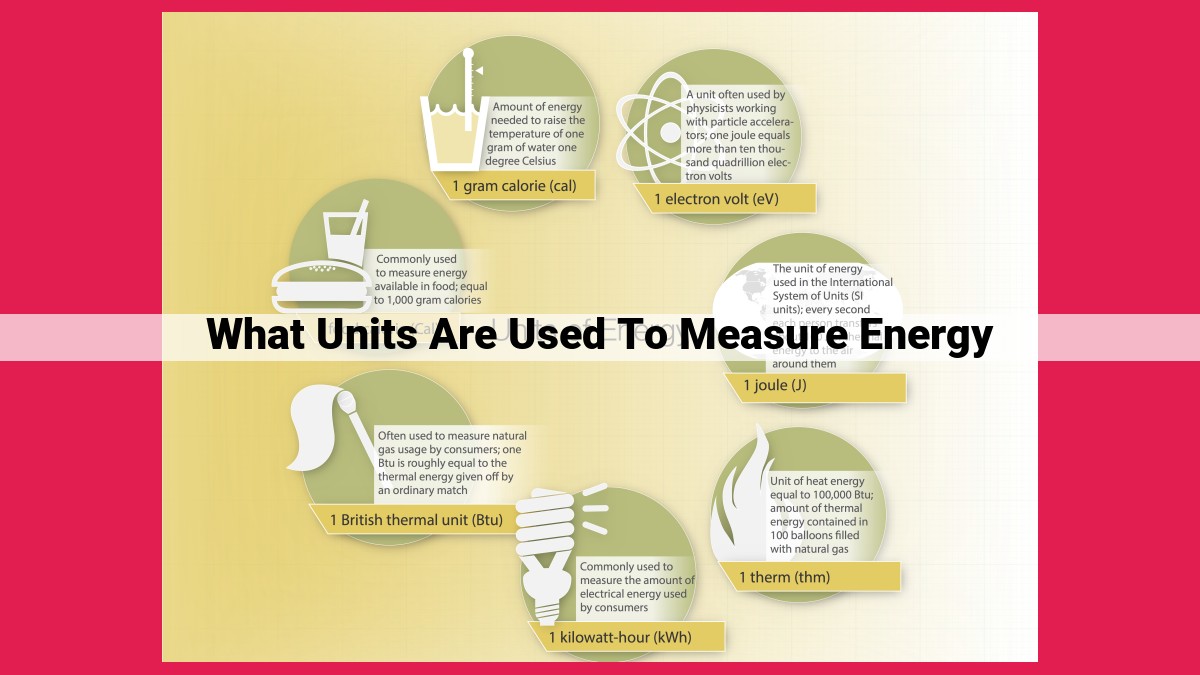
Energy is measured in various units, each tailored to specific applications. The joule (J) measures mechanical energy, the calorie (cal) measures thermal energy, the kilocalorie (kcal) is used in nutritional labeling, and the electronvolt (eV) measures particle energy in physics. Understanding these units is essential for accurate quantification and analysis in fields like engineering, physics, biology, and nutrition.
Measuring Energy: Units Explored
Energy, the fundamental power that drives our world, is an abstract concept that requires precise measurement for us to comprehend and harness its potential. Throughout history, scientists have devised various units to quantify this enigmatic force, each tailored to specific scientific disciplines and everyday applications.
In this article, we embark on a captivating exploration of these units, unraveling their historical evolution, practical significance, and how they empower us to measure and understand the world around us. From the humble origins of the joule to the exotic realms of particle physics, we will delve into the fascinating stories behind these units, shedding light on their intricate relationship with energy. By mastering these units, we gain a deeper appreciation for the power and elegance of scientific inquiry.
The Joule: Work, Force, and Distance: Unraveling the Unit of Energy in Mechanical Systems
In the realm of physics, energy reigns supreme as the fundamental currency of change and transformation. To harness and quantify this enigmatic force, scientists have devised a myriad of units, each tailored to specific scientific and technological domains. Among these units, the joule stands tall as the cornerstone of energy measurement in the world of mechanical systems.
The joule, named after the esteemed physicist James Prescott Joule, is defined as the work performed when a force of one newton is applied over a distance of one meter. This simple yet profound concept encapsulates the essence of mechanical energy, providing a precise measure of the ability to do work.
Work itself is the transfer of energy from one object to another, typically through the application of a force. Imagine a weightlifter hoisting a heavy barbell overhead. The force exerted by the lifter’s muscles against the barbell, combined with the distance the barbell travels upward, multiplies to yield the total work done.
Force, on the other hand, represents a push or pull that acts upon an object, causing it to accelerate or deform. The classic example is the force of gravity that keeps us firmly planted on the ground.
Distance, as we all know, is the measure of the length or separation between two points. In the context of mechanical energy, distance plays a crucial role in determining the amount of work performed. A longer distance traversed by an object subjected to a force translates into a greater amount of work accomplished.
To illustrate the practical application of the joule, consider a simple machine like a lever. When a force is applied to one end of the lever, it creates a torque, causing the other end to move. The amount of work done by the lever is determined by the force applied, the distance moved, and the lever’s efficiency.
In the realm of engines and power plants, the joule is an indispensable unit for quantifying energy conversion. The power of an engine, measured in joules per second, represents the rate at which it converts fuel into mechanical energy.
Understanding the joule and its relationship with work, force, and distance is essential for grasping the fundamental principles of mechanics and for accurately quantifying energy in a wide range of mechanical systems. From the simple operation of levers to the intricate workings of engines, the joule empowers us to measure, analyze, and harness the power of mechanical energy.
The Calorie: Unveiling the Energy of Heat, Temperature, and Water
In the vast tapestry of energy measurement, there exists a unit that plays a pivotal role in understanding the thermal realm: the calorie. Defined as the amount of heat energy required to raise the temperature of one gram of pure water by one degree Celsius, the calorie is a cornerstone concept in thermal science.
The calorie’s significance stems from its ability to quantify the energy involved in thermal processes. Just as a joule measures the energy of mechanical systems, the calorie measures the energy associated with heat and temperature.
The Calorie’s Thermal Journey
Imagine a cup of water nestled on a stovetop. As the burner ignites, sending waves of heat to the water, its temperature gradually rises. Each calorie of energy absorbed by the water represents a tiny increment in its thermal energy. This heat energy is what powers the water’s transformation from a cold liquid to a boiling one.
The Calorie in Nutrition
Beyond its thermal applications, the calorie also holds a prominent place in the world of nutrition. The kilocalorie (kcal), a unit equal to 1,000 calories, is commonly used on food labels to indicate the energy content of foods.
Understanding the Calorie’s Impact
Grasping the concept of the calorie is crucial for navigating the world of thermal energy and nutritional science. It empowers us to comprehend the energy dynamics involved in everyday occurrences, from the warmth of our homes to the sustenance we derive from food.
The calorie is a fundamental unit of energy measurement, providing a window into the thermal and nutritional aspects of our world. By understanding the calorie, we gain a deeper appreciation for the interplay between energy and our physical experiences.
The Kilocalorie: Energy in Food
In the world of nutrition, kilocalories, often abbreviated as kcal, play a crucial role in quantifying the energy content of food. Understanding this unit of measurement is essential for making informed choices about our dietary intake.
A kilocalorie is equal to 1,000 calories, a unit that measures heat energy. When we consume food, the body converts it into energy through a complex process known as metabolism. The energy released during this process is used to power our bodily functions, such as maintaining body temperature, performing physical activities, and repairing tissues.
Food labels typically display energy content in kilocalories per serving. This information helps us understand how much energy we’re consuming from a particular food. For example, a 100-gram serving of pasta may contain 150 kcal, while a 30-gram serving of almonds may provide 160 kcal.
The daily recommended calorie intake varies based on factors such as age, gender, and activity level. However, it’s generally recommended that adult women consume around 2,000 kcal per day, while adult men require approximately 2,500 kcal.
Understanding kilocalories empowers us to make informed choices about our diet. By comparing the energy content of different foods, we can ensure we’re consuming a balanced diet that meets our individual needs. It also helps us manage our weight, as consuming more calories than we burn leads to weight gain, while burning more calories than we consume results in weight loss.
Tracking kilocalories can be beneficial for individuals with specific dietary goals, such as weight loss, muscle building, or managing chronic diseases. It allows us to monitor our energy intake and make adjustments as needed.
The Electronvolt: Delving into the Energy of Particles
In the realm of particle physics, where the smallest building blocks of matter collide, energy plays a pivotal role. To measure the minute amounts of energy involved in these subatomic interactions, scientists rely on a specialized unit known as the electronvolt (eV).
At its core, the electronvolt is defined as the energy acquired by an electron when it is accelerated through a potential difference of one volt. This fundamental unit forms the cornerstone of particle physics measurements, providing a precise way to quantify the energy of tiny particles like electrons, protons, and even entire atoms.
In practical terms, the electronvolt often appears in conjunction with larger prefixes, such as mega- (MeV) or giga- (GeV), to accommodate the vast energies encountered in particle accelerators. For instance, the Large Hadron Collider, the world’s most powerful particle accelerator, operates at energies exceeding 13 teraelectronvolts (13 TeV), allowing scientists to probe the fundamental nature of the universe.
The electronvolt’s significance extends beyond particle physics. It also finds applications in fields such as nuclear physics, where it measures the energy released during nuclear reactions, and in the study of semiconductors, where it determines the energy levels of electrons within electronic devices.
By understanding the electronvolt and its role in measuring particle energy, we gain a deeper appreciation for the fundamental forces that govern the smallest particles in our universe. This knowledge empowers scientists to explore the realm of particle physics, unlocking the secrets of the cosmos and advancing our understanding of the fundamental building blocks of matter.
Contextualizing the Energy Units
In the realm of science and technology, energy takes center stage. Precise quantification of energy is crucial to comprehend the workings of the universe and drive advancements. To cater to diverse applications, a tapestry of energy units has been woven, each tailored to specific contexts.
The joule, the SI unit of energy, embodies the interplay of work, force, and distance. It paints a vivid picture of energy as the ability to perform work, whether it’s lifting a weight, pushing an object, or overcoming friction. In the arena of mechanics, the joule reigns supreme, enabling engineers to design efficient machines, calculate the energy stored in springs and flywheels, and unravel the secrets of motion.
Stepping into the world of thermal systems, we encounter the calorie. This unit captures the energy involved in heating substances, particularly water. From measuring the warmth of food to determining the efficiency of heating appliances, the calorie plays a vital role in understanding and controlling thermal processes.
In the domain of nutrition, the kilocalorie takes the spotlight. This larger unit, often used interchangeably with the calorie, quantifies the energy content of food. Dieticians and health enthusiasts rely on kilocalories to devise balanced diets, optimize weight management, and fuel their bodies for peak performance.
Delving into the subatomic realm, we encounter the electronvolt. This unit measures the energy of particles, particularly electrons. It’s an indispensable tool in particle physics, allowing scientists to probe the mysteries of atomic and nuclear reactions, unravel the secrets of quantum mechanics, and push the boundaries of our understanding of the universe.