Mastering Unit Conversions: Converting Atoms To Grams With Precision

To convert from atoms to grams, you must understand the relationships between atomic mass, molar mass, Avogadro’s number, and mass. The atomic mass of an element is the mass of one atom, while the molar mass of a substance is the mass of one mole of that substance. Avogadro’s number is the number of atoms in one mole of a substance, approximately 6.022 x 10^23. Using these relationships, you can convert from the number of atoms to moles by dividing by Avogadro’s number. Then, multiply the number of moles by the molar mass to obtain the mass in grams.
From Atoms to Grams: Unveiling the Secrets of Chemical Quantities
In the realm of chemistry, understanding the relationships between atomic mass, molar mass, Avogadro’s number, and mass is crucial for navigating the microscopic world and making sense of the macroscopic properties of matter. This comprehensive guide will take you on a journey from the fundamental building blocks of matter, atoms, to the measurable quantities we use in chemistry, grams.
Atomic Mass: The Weight of a Single Atom
At the heart of every element lies the concept of atomic mass, the mass of a single atom of that element. Measured in atomic mass units (amu), it represents the combined weight of the protons, neutrons, and electrons that make up the atom.
Relationship with Molar Mass, Avogadro’s Number, and Mass
Atomic mass plays a pivotal role in determining two other fundamental quantities: molar mass and Avogadro’s number. Molar mass, expressed in grams per mole (g/mol), is the mass of one mole of a substance, which is the amount containing exactly Avogadro’s number of atoms or molecules. Avogadro’s number, a colossal 6.022 x 10^23, is the number of atoms or molecules present in one mole of any substance.
Calculating Mass and Number of Atoms
Understanding atomic mass allows us to calculate the mass and number of atoms in a given sample. By multiplying the number of atoms by the atomic mass, we can determine the mass of the sample. Conversely, knowing the mass and atomic mass lets us calculate the number of atoms present.
Example
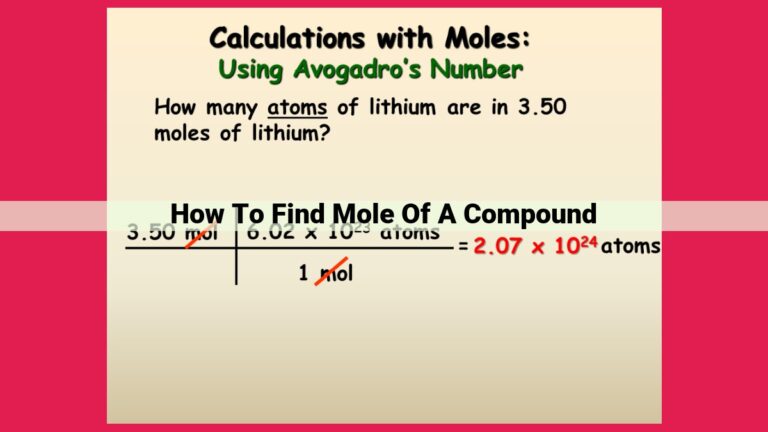
For instance, the atomic mass of carbon is 12 amu. One mole of carbon weighs 12 g, and contains 6.022 x 10^23 carbon atoms. If we have a sample of carbon with a mass of 6 g, we can calculate the number of carbon atoms by dividing the mass by the atomic mass: 6 g / 12 g/mol = 0.5 moles. Then, we can multiply the number of moles by Avogadro’s number: 0.5 moles x 6.022 x 10^23 atoms/mol = 3.011 x 10^23 atoms.
Molar Mass: The Foundation of Chemical Stoichiometry
In the realm of chemistry, we navigate the microscopic world of atoms and molecules, where understanding the interplay between their masses is crucial. Molar mass, a fundamental concept, serves as a bridge between the atomic and macroscopic scales, enabling us to unravel the relationships between the minuscule building blocks of matter and the quantities we can measure in the lab.
Defining Molar Mass
Molar mass is defined as the mass of one mole of a substance. A mole is an enormous unit, representing a whopping 6.022 × 10^23 individual entities, whether atoms, molecules, or ions. By assigning a molar mass to each substance, we establish a universal scale that allows us to compare their masses and determine their relative abundance in chemical reactions.
The Interdependence of Atomic Mass, Avogadro’s Number, and Molar Mass
Molar mass is intricately connected to the atomic mass of an element, which represents the average mass of its atoms. However, atomic masses are expressed in atomic mass units (amu), an inconveniently small unit for practical purposes.
Enter Avogadro’s number, the magical constant that bridges the atomic and macroscopic worlds. It represents the number of entities present in one mole of a substance. By multiplying the atomic mass of an element by Avogadro’s number, we obtain its molar mass, expressed in grams per mole (g/mol).
Molar Mass in Action
The versatility of molar mass extends beyond theoretical understanding. It finds practical applications in various chemical calculations:
-
Converting between Mass and the Number of Moles: By knowing the molar mass of a substance, we can easily convert between its mass and the number of moles present.
-
Determining the Number of Entities: Avogadro’s number empowers us to calculate the precise number of atoms, molecules, or ions in a given sample by dividing its mass by its molar mass.
-
Stoichiometric Calculations: Molar mass is indispensable in stoichiometry, the branch of chemistry that deals with quantitative relationships in chemical reactions. By using molar masses, we can determine the exact amounts of reactants and products involved in a reaction, ensuring balanced equations and precise predictions.
In essence, molar mass serves as a pivotal tool in chemistry, enabling us to comprehend the composition of matter, perform accurate calculations, and predict the outcomes of chemical reactions with confidence.
Avogadro’s Number: The Bridge Between the Microscopic and Macroscopic Worlds
In the vast realm of chemistry, where the tiniest particles dance and interact, lies the enigmatic concept of Avogadro’s number. This fundamental constant serves as a crucial link between the microscopic world of atoms and the macroscopic world we experience daily.
What is Avogadro’s Number?
Avogadro’s number, denoted by the symbol N_a, is the number of atoms contained within a single mole of any substance. A mole is a fundamental unit used in chemistry to measure the quantity of a substance. It is defined as the amount of a substance that contains N_a atoms. This staggering number, approximately 6.022 x 10^23, provides a bridge between the microscopic and macroscopic scales.
Connecting the Dots: Avogadro’s Number and Other Chemical Quantities
Avogadro’s number is intricately connected to other essential chemical quantities, such as atomic mass and molar mass. Atomic mass is the mass of a single atom of an element, while molar mass is the mass of one mole of a substance.
The relationship between these quantities can be expressed through the following formula:
Molar mass = Atomic mass x _N_a_
This formula highlights the role of Avogadro’s number in converting between the atomic scale and the macroscopic scale. By multiplying the atomic mass of an element by N_a, we obtain the molar mass, which is the mass of a specific number of atoms (precisely N_a atoms) expressed in grams.
Calculating Atoms and Moles Using Avogadro’s Number
Avogadro’s number also empowers chemists to determine the number of atoms or moles in a given sample. The number of atoms can be calculated by multiplying the number of moles by N_a, while the number of moles can be calculated by dividing the number of atoms by N_a.
These calculations are vital for performing stoichiometric calculations, which involve balancing chemical equations to predict the quantities of reactants and products involved in a particular reaction.
Avogadro’s Number: A Gateway to the Chemical World
Armed with the knowledge of Avogadro’s number, chemists can navigate the complexities of the chemical world with greater ease. This enigmatic constant enables them to bridge the microscopic and macroscopic scales, making it possible to understand the behavior of matter at both the atomic and molecular levels.
The Intriguing Relationship between Mass, Atomic Mass, and Molar Mass
In our scientific quest to understand the world around us, we often encounter the concepts of mass, atomic mass, and molar mass. These terms might seem daunting at first, but they are essential for comprehending the nature of matter. Let’s embark on a storytelling journey to unravel their interconnectedness.
Mass: The Essence of Matter
Mass, the physical property of matter, represents the quantity of matter it contains. It serves as the foundation for understanding the weight and density of substances. Mass, measured in grams, is a fundamental aspect of every object, from the smallest atoms to the largest celestial bodies.
Atomic Mass: The Building Blocks of Mass
Atoms, the fundamental units of matter, possess a unique characteristic known as atomic mass. This value represents the average mass of one atom of an element. Atomic mass is expressed in atomic mass units (amu) or Daltons (Da). Understanding atomic mass helps us determine the relative heaviness of elements and their isotopes.
Molar Mass: The Bridge between Mass and Amount
Molar mass is a concept that links mass to the amount of substance. It represents the mass of one mole of a substance, which is defined as 6.022 × 10^23 particles (atoms, molecules, or ions). The molar mass, measured in grams per mole (g/mol), provides a crucial connection between the mass of a substance and the number of particles it contains.
Unraveling the Interconnections
The relationship between mass, atomic mass, and molar mass is intricate but essential. Mass is the total amount of matter in a sample, while atomic mass is the average mass of one atom of an element. Molar mass bridges the gap by defining the mass of one mole of a substance, which is a specific number of particles.
To illustrate the interdependence, let’s consider an example. Carbon-12, an isotope of carbon, has an atomic mass of 12 amu. If we have 6.022 × 10^23 atoms of carbon-12 (one mole), the mass of this sample would be 12 grams. This is because the molar mass of carbon-12 is 12 g/mol, indicating that 1 mole of carbon-12 has a mass of 12 grams.
The concepts of mass, atomic mass, and molar mass are intertwined, providing a framework for understanding the nature of matter. Whether we are exploring the intricacies of chemical reactions or simply measuring the weight of everyday objects, these concepts serve as essential tools for scientific inquiry.
Number of Atoms
Determining the number of atoms in a substance is crucial for various calculations in chemistry. It is directly related to Avogadro’s number and the number of moles.
Avogadro’s number, a fundamental constant in chemistry, represents the number of atoms in one mole of a substance. It serves as a bridge between the macroscopic and microscopic worlds. By multiplying the number of moles by Avogadro’s number, we can determine the number of atoms present.
For instance, if we have 3 moles of sodium (Na), we can calculate the number of atoms as follows:
Number of atoms = Number of moles × Avogadro's number
Number of atoms = 3 moles × 6.022 × 10^23 atoms/mole
Number of atoms = 1.8066 × 10^24 atoms
This result indicates that 3 moles of sodium contain a whopping 1.8066 × 10^24 atoms!
Understanding the interrelationship between these concepts is essential for navigating the fascinating world of chemistry. By mastering these principles, you can confidently explore the composition and properties of matter.
Number of Moles: The Connecting Link
In the realm of chemistry, understanding the number of moles is crucial, as it serves as the bridge between the microscopic world of atoms and the macroscopic world of grams. Moles, like a magic wand, connect these two dimensions, allowing us to seamlessly convert between the number of atoms and the mass of a substance.
The number of moles is symbolized by the letter n, and it’s defined as the amount of substance that contains exactly 6.022 x 10^23 fundamental units. These units can be atoms, molecules, ions, or electrons, depending on the substance in question.
To convert between the number of moles (n), the molar mass (M), and the number of atoms (N), we rely on Avogadro’s number (Nₐ), which acts as the conversion factor:
- n = N/Nₐ (converting atoms to moles)
- N = n x Nₐ (converting moles to atoms)
Molar mass, represented by the symbol M, is the mass of one mole of a substance, expressed in grams per mole (g/mol). It provides a direct link to the mass of a substance:
- Mass = n x M
Understanding the interrelationship between these concepts empowers us to perform essential calculations, such as determining the number of moles in a given mass of a substance, calculating the mass of a known number of atoms, or converting between the number of atoms and the number of moles.
By mastering these conversions, you’ll unlock the ability to navigate the microscopic and macroscopic worlds of chemistry with ease, making you a true alchemist of knowledge!