Understanding Radioactive Isotopes: Applications And Implications
Radioactive isotopes differ from isotopes by their unstable nuclei, which emit radiation to achieve stability. While isotopes are variants of an element with varying neutron count, radioactive isotopes exhibit an imbalance in their proton-neutron ratio, resulting in spontaneous radioactive decay. The process of decay follows a specific time interval called half-life, allowing scientists to measure the rate of decay. Radioactive isotopes find applications in medical imaging, cancer therapy, archaeological dating, industrial tracing, and scientific research.
Understanding Isotopes: The Basics
- Define isotopes as variants of an element with varying neutron count.
- Explain how protons define atomic number and element identity.
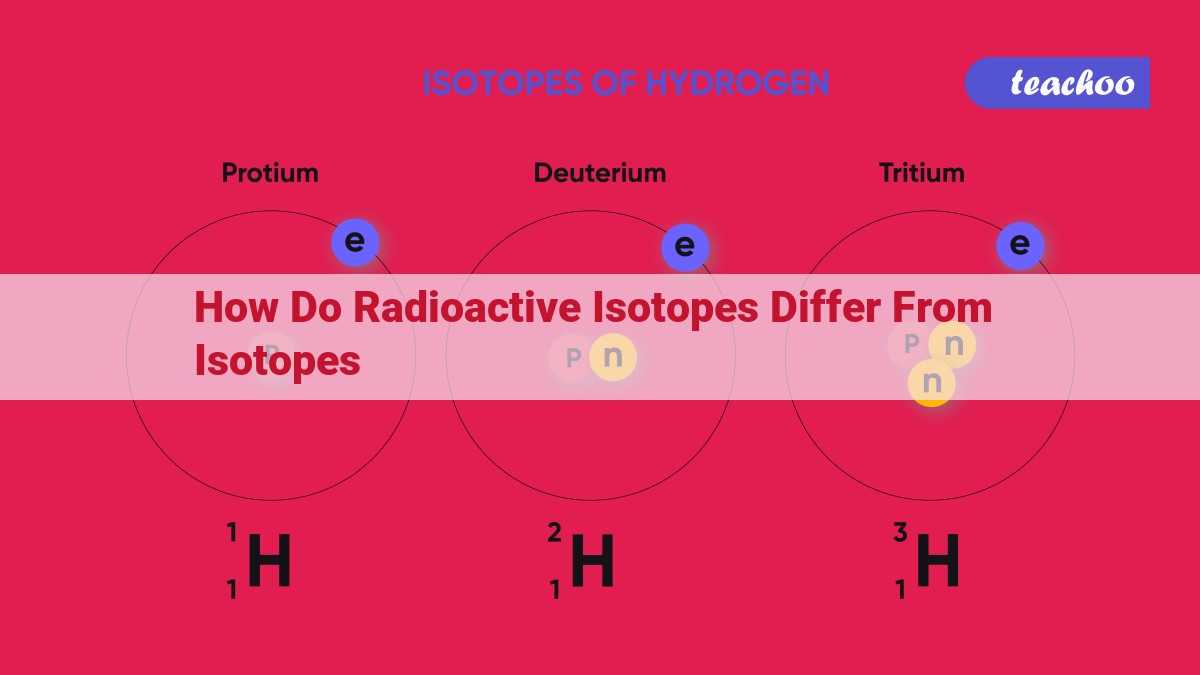
Understanding Isotopes: Unveiling the Building Blocks of Matter
In the captivating world of chemistry, the atom reigns supreme, composed of the enigmatic particles known as protons, neutrons, and electrons. Protons, the gatekeepers of an element’s identity, dictate its atomic number and its place on the periodic table. Enter isotopes, fascinating variants of an element that share the same atomic number but differ in their neutron count.
Think of isotopes as twins: they have the same fundamental characteristics of their parent element but possess a distinct number of neutrons. This difference in neutron count alters the mass of the isotope without affecting its atomic number or chemical properties. For instance, the element carbon exists as three isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12, the most abundant isotope, has six protons and six neutrons. Carbon-13 boasts an extra neutron, while carbon-14, a radioactive isotope, has two additional neutrons.
Protons, the positively charged particles, reside within the atom’s core alongside neutrons. Neutrons, true to their name, lack an electrical charge and serve as a balancing force, stabilizing the nucleus. A harmonious balance between protons and neutrons is the key to atomic stability. However, when this equilibrium is disrupted, the atom becomes unstable, leading to the creation of radioactive isotopes.
Stability and Radioactive Isotopes: Understanding Nuclear Unrest
In the realm of atoms, where protons and neutrons dance in harmony, we encounter a fascinating duality: stable isotopes and radioactive isotopes. Let’s delve into this intriguing world and uncover their secrets.
Stable Isotopes: A Perfect Balance
Imagine a tranquil lake, where protons (positively charged) and neutrons (no charge) coexist in perfect equilibrium. These are stable isotopes. Their proton-neutron ratios are so harmoniously balanced that they remain content, with no urge to change. They form the building blocks of ordinary matter, from the air we breathe to the rocks beneath our feet.
Radioactive Isotopes: Born with a Nuclear Itch
But not all isotopes are so serene. Some harbor an inner turmoil, an imbalance within their nuclear structure that compels them to seek stability. These are radioactive isotopes. Their proton-neutron ratios are off-kilter, creating a restless energy within their nuclei.
Driven by this instability, radioactive isotopes have an inherent tendency to release radiation, a form of energy that can take various shapes: alpha particles (helium nuclei), beta particles (electrons or positrons), or gamma rays (penetrating electromagnetic waves). By emitting radiation, radioactive isotopes strive to attain a more stable configuration, like a restless spirit seeking tranquility.
Half-Life: Unraveling the Secrets of Radioactive Decay
When atoms possess an excess of energy, they embark on a journey of transformation, shedding this surplus through a process known as radioactive decay. This decay is nature’s way of restoring stability, as atoms strive to achieve an equilibrium between protons and neutrons within their nuclei.
Concept of Half-Life
A crucial concept in understanding radioactive decay is half-life. Half-life represents the duration it takes for half of the radioactive atoms in a sample to decay. This decay occurs at a constant rate, meaning that the number of atoms decaying per unit time is proportional to the total number of radioactive atoms present.
Significance of Half-Life
Half-life plays a pivotal role in measuring radioactive decay. It allows scientists to determine the activity of a radioactive sample, which indicates the rate at which atoms are decaying. By knowing the half-life and activity, researchers can calculate the age of radioactive materials, such as fossils and archaeological artifacts, through a technique called radioactive dating.
Applications in Medicine and Research
Half-life finds practical applications in the field of medicine. For instance, the half-life of iodine-131 is utilized in treating thyroid cancer. This radioactive isotope emits radiation that selectively targets and destroys cancerous cells.
Moreover, half-life is essential in research. By studying the half-lives of different isotopes, scientists gain valuable insights into the structure of atoms and the mechanisms of decay. This knowledge contributes to advancements in nuclear physics and other areas of scientific investigation.
Applications of Radioactive Isotopes: Unlocking Unseen Benefits
Radioactive isotopes, once shrouded in mystery, have emerged as indispensable tools in a wide array of fields, from medicine to archaeology, and even industrial research. These isotopes, with their ability to decay and emit radiation, hold the key to unlocking hidden knowledge and advancing human progress.
Healing and Diagnosis: The Medical Marvels of Radioactive Isotopes
In the realm of medicine, radioactive isotopes have revolutionized medical imaging and cancer therapy. Technetium-99m, for example, is a widely used isotope that emits gamma rays, allowing doctors to create detailed images of organs and tissues. This non-invasive technique aids in diagnosing various diseases, from heart conditions to neurological disorders.
Isotopes also play a pivotal role in cancer therapy. By precisely targeting cancerous cells, Cobalt-60 emits high-energy gamma rays that destroy tumor tissue with minimal harm to healthy cells. Iodine-131 is another isotope used to treat thyroid cancer.
Unraveling Time’s Secrets: Applications in Archaeology and Geology
Archaeologists and geologists rely on radioactive isotopes to determine the age of ancient artifacts and fossils. Carbon-14 is a prime example, providing insights into the age of organic materials up to 50,000 years old. Potassium-40 and Argon-40 help unravel the mysteries of rocks and geological formations, extending our understanding of Earth’s history over millions of years.
Industrial Applications: Tracing and Testing with Isotopes
In the industrial realm, radioactive isotopes offer valuable tools for tracing and material testing. Iridium-192 is used to monitor the flow of liquids and gases in pipelines. Gamma radiography, using isotopes like Cobalt-60, provides intricate images of internal structures, aiding in quality control and defect detection.
Scientific Research: Unlocking the Secrets of Atomic Structure and Decay
Radioactive isotopes also play a crucial role in scientific research. By studying their decay mechanisms, scientists gain insights into the structure of atoms and the interactions that govern them. These isotopes provide valuable data for understanding nuclear physics and developing new radioactive isotopes with specific properties for various applications.