Understanding The Impact Of Pyrimidine Dimers On Dna Health And Disease
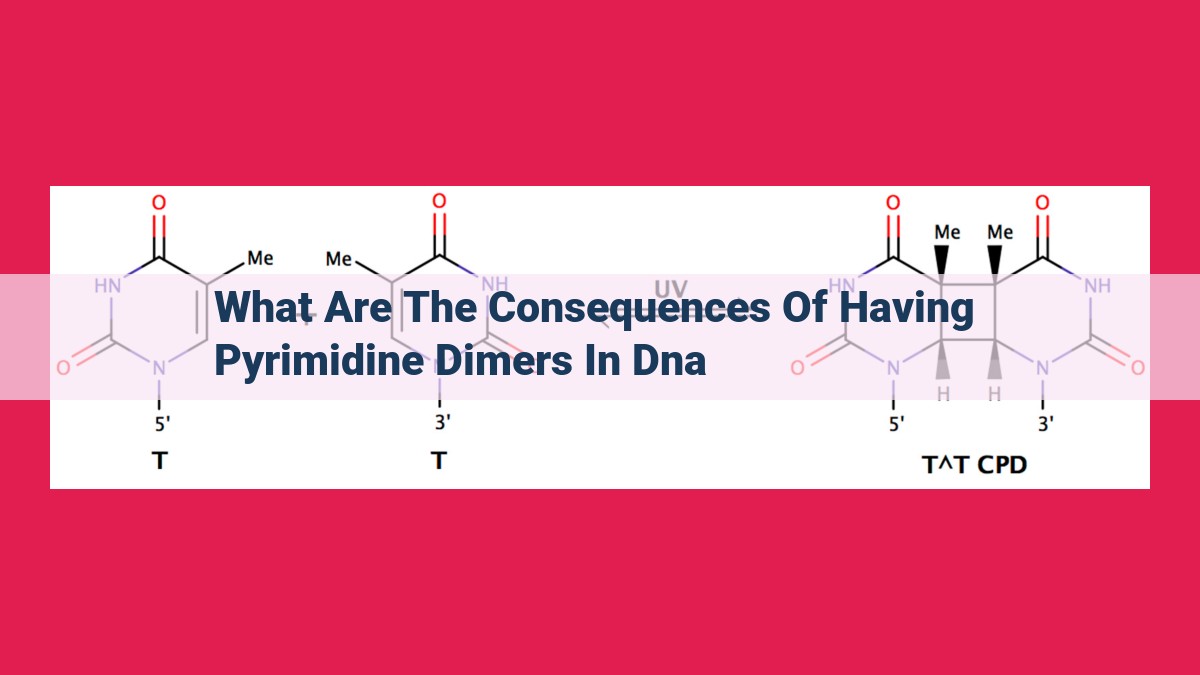
Pyrimidine dimers, lesions that occur in DNA after UV exposure, have significant consequences: hinder DNA replication causing replicative stress, induce mutations through error-prone bypass, trigger cell death pathways including apoptosis, and disrupt cellular processes. These effects compromise genome integrity and cell viability, contributing to disease development. Repair mechanisms, such as nucleotide excision repair and photoreactivation, are crucial for mitigating the detrimental outcomes of pyrimidine dimers.
- Definition and formation of pyrimidine dimers
- Significance of these lesions for DNA structure and function
Pyrimidine Dimers: Guardians of the Genome, Sentinels of the Sun
We live in a world bathed in sunlight, a celestial tapestry that nourishes life. Yet, nestled within its golden rays lurks an invisible adversary—ultraviolet (UV) radiation. This high-energy force can penetrate the shields of our cells, wreaking havoc on our genetic blueprint: our DNA. Enter pyrimidine dimers, unsung heroes that tirelessly guard our genome from the sun’s insidious assault.
Pyrimidine dimers are distorted DNA structures that form when two adjacent pyrimidine bases—the building blocks of our genetic code—become irreversibly linked under the onslaught of UV radiation. These dimers, like tiny molecular shackles, disrupt the smooth flow of DNA replication, the fundamental process of genetic inheritance. They hinder the progress of DNA polymerase, the enzyme responsible for copying our genetic blueprint, leading to a cascade of events that can threaten the integrity of our genome.
The presence of pyrimidine dimers wreaks havoc on cellular processes. DNA replication, transcription, and other essential functions are compromised, leading to a disruption in the delicate balance of life. This disturbance can trigger replicative stress, a cellular alarm bell that signals the presence of unrepaired damage. Replicative stress, if left unaddressed, can lead to the formation of single- and double-strand breaks in our precious DNA, wounds that can cripple our cells and contribute to disease development.
Replicative Stress Induced by Pyrimidine Dimers
Pyrimidine dimers, obstacles in the DNA’s tightly-knit structure, can severely impair DNA replication. Imagine a vital highway obstructed by immovable barricades, and you’ll understand the plight of DNA polymerases, which cannot bypass these formidable roadblocks.
-
Stalled Replication Fork: As DNA polymerase attempts to navigate the DNA landscape, it encounters pyrimidine dimers. The distorted DNA strand becomes an insurmountable barrier, halting DNA replication at its tracks.
-
Replicative Stress: The replication impediment triggers replicative stress, which can have devastating consequences for DNA integrity. Cells respond to this stress by activating various pathways to circumvent stalled replication forks.
-
Single- and Double-Strand Breaks: Replicative stress can lead to genome instability, including the formation of single-strand breaks (SSBs) and double-strand breaks (DSBs). These breaks are potentially lethal, causing DNA damage and increasing the likelihood of cell death.
Pyrimidine dimers are a serious threat to the health of cells, wreaking havoc on their ability to faithfully replicate their genetic material. Understanding their deleterious effects on DNA replication is crucial for safeguarding our health and protecting ourselves from the harmful effects of environmental insults.
The Devastating Mutagenic Effects of Pyrimidine Dimers: Unraveling the Threat to Our Cellular Blueprint
Pyrimidine dimers, insidious lesions formed when ultraviolet (UV) radiation strikes DNA, pose a grave threat to our genetic integrity and cellular well-being. These twisted structures disrupt the precise double-helix architecture of DNA, hindering replication and introducing mutations that can unravel the very fabric of our genetic code.
Error-Prone Replication: As the cellular machinery attempts to copy damaged DNA strands, the presence of pyrimidine dimers creates insurmountable obstacles. DNA polymerases, the enzymes responsible for copying DNA, often stall at these lesions, leading to replication stuttering. To overcome this impasse, cells may resort to error-prone bypass, a reckless attempt to continue replication despite the presence of distortions. This reckless approach introduces incorrect nucleotides into the newly synthesized DNA strand, creating mutations that can alter gene function and potentially trigger disease.
Genetic Instability and Disease Development: The accumulation of unrepaired pyrimidine dimers disrupts the intricate mechanisms that maintain genome stability, leading to a perilous state of genetic chaos. These mutations can disrupt crucial cellular processes, from cell division to DNA repair itself, creating a vicious cycle of genomic instability. Over time, this instability can drive the development of a wide spectrum of diseases, including skin cancer, neurodegenerative disorders, and immune system dysfunction.
The far-reaching consequences of pyrimidine dimer-induced mutagenesis underscore the critical importance of protecting ourselves from harmful UV radiation. By understanding the mutagenic effects of these insidious lesions, we can appreciate the vital role of preventive measures such as sunscreen and protective clothing in safeguarding our genetic heritage and promoting cellular health.
Unveiling the Deadly Toll of Pyrimidine Dimers: Cell Death Pathways
Pyrimidine dimers, insidious DNA lesions caused by ultraviolet radiation, wreak havoc upon cellular machinery. Their presence triggers a cascade of events, ultimately leading to cell death. Let’s delve into the two primary pathways involved: apoptosis and necrosis.
Apoptosis: A Programmed Demise
When unrepaired pyrimidine dimers accumulate, cells initiate a programmed death process known as apoptosis. This highly regulated pathway ensures the removal of damaged or dysfunctional cells without harming neighboring cells.
The process begins with mitochondrial dysfunction__, releasing pro-apoptotic proteins. These proteins activate **caspases, enzymes that methodically dismantle the cell from within. Apoptosis is characterized by distinct morphological changes, such as cell shrinkage, nuclear fragmentation, and the formation of apoptotic bodies.
Necrosis: Uncontrolled Destruction
In contrast to apoptosis, necrosis is an unprogrammed form of cell death that occurs when cellular damage is severe. Unrepaired pyrimidine dimers disrupt essential cellular functions, causing a breakdown of homeostasis leading to membrane rupture and the release of cellular contents.
Necrosis is characterized by cell swelling, loss of membrane integrity, and the absence of the organized morphological changes seen in apoptosis. This uncontrolled cell death can trigger inflammation and damage surrounding tissues.
Cellular Mechanisms: Detecting and Eliminating Damage
Cells possess mechanisms to detect and repair pyrimidine dimers and other DNA lesions. However, when the damage is extensive or persistent, cell death pathways are activated.
Key players in apoptosis include the Bcl-2 family of proteins (regulating mitochondrial integrity) and caspases. In necrosis, the activation of PARP-1 and the release of S100 protein contribute to cell destruction.
Consequences for Cellular Health
Pyrimidine dimer-induced cell death has profound implications for cellular health and disease development. Unrepaired dimers can lead to genetic instability and the accumulation of DNA damage, increasing the risk of cancer and other degenerative diseases. Moreover, excessive cell death can disrupt tissue function and contribute to immune dysfunction.
Significance for UV Protection
Understanding the cell death pathways triggered by pyrimidine dimers underscores the importance of UV protection. By employing sunscreen, protective clothing, and other preventative measures, we can shield our cells from the damaging effects of UV radiation and reduce the risk of DNA damage, cell death, and associated health consequences.
Mechanisms for Repairing Pyrimidine Dimers: Safeguarding Our Genetic Blueprint
Pyrimidine Dimers: The Silent Threat to DNA Health
Pyrimidine dimers, mischievous molecular miscreants, pose a significant threat to the integrity of our genetic material. These pesky lesions, formed when adjacent pyrimidine bases on the DNA strand become crosslinked by ultraviolet radiation, disrupt the delicate dance of DNA replication and cellular function.
Repair Mechanisms to the Rescue
Fortunately, our cells have evolved ingenious repair mechanisms to combat these dimeric disruptors. Two key players in this DNA damage control mission are nucleotide excision repair (NER) and photoreactivation.
Nucleotide Excision Repair (NER): Precision Surgery for Damaged DNA
NER is a surgical repair process that meticulously identifies and removes the damaged DNA segment containing the pyrimidine dimer. It employs a team of specialized enzymes to snip out the afflicted region and replace it with a pristine DNA stretch, restoring the genetic code to its original glory.
Photoreactivation: Harnessing Sunlight for DNA Healing
Photoreactivation, on the other hand, utilizes sunlight as its weapon against pyrimidine dimers. This enzymatic process leverages the power of light to break the crosslinking bonds between the dimerized pyrimidines, effectively restoring the DNA’s structural integrity.
Importance of Repair Mechanisms
These repair mechanisms are crucial for maintaining the integrity of our genetic code. Without their diligent efforts, pyrimidine dimers would accumulate, wreaking havoc on cellular processes and potentially leading to disease development. Therefore, understanding and preserving these repair mechanisms are vital for our well-being and the health of our genetic heritage.
Consequences of Pyrimidine Dimers on Cellular Processes
Pyrimidine dimers, insidious DNA lesions caused by UV radiation, pose a significant threat to cellular well-being. These lesions disrupt the fundamental processes that underpin DNA’s vital functions, leading to a cascade of harmful consequences that can cripple cell viability and contribute to disease pathogenesis.
Disruption of Essential Cellular Functions
Pyrimidine dimers act as formidable roadblocks to the smooth flow of cellular operations. DNA replication, the meticulous process of copying DNA, is severely hindered by these lesions. DNA polymerases, the molecular machines that synthesize new DNA strands, stutter and falter upon encountering these roadblocks, leading to replication stress. This stress can manifest as single- and double-strand breaks, potentially catastrophic events that can derail the orderly progression of DNA duplication.
The consequences extend beyond DNA replication. Transcription, the essential process of converting DNA into messenger RNA (mRNA), is also disrupted by pyrimidine dimers. This disruption can lead to the production of defective mRNA, which in turn impairs protein synthesis and compromises cellular function. Other vital cellular processes, such as DNA repair, recombination, and chromatin remodeling, also suffer from the presence of these DNA lesions.
Impact on Genome Integrity and Cell Viability
The unremitting assault of pyrimidine dimers on essential cellular functions has a profound impact on genome integrity. DNA damage, if left unrepaired, can lead to mutations and chromosomal aberrations. These genetic alterations can give rise to cancerous cells and promote the development of debilitating diseases. The compromised genome integrity also weakens cells, making them more susceptible to apoptosis, a form of programmed cell death, and necrosis, an uncontrolled and often irreversible form of cell death.
The accumulation of unrepaired pyrimidine dimers can overwhelm the cellular machinery, leading to a state known as replicative senescence. Senescent cells cease to divide, contributing to tissue aging and age-related diseases. Additionally, persistent DNA damage can trigger the activation of immune responses, further exacerbating cellular dysfunction and ultimately compromising cell viability.
Pyrimidine dimers are not mere bystanders in the cellular landscape. They are formidable disruptors that leave an indelible mark on DNA structure and function, leading to a cascade of harmful consequences that can compromise genome integrity, impair cell viability, and promote disease development. Understanding these consequences is paramount in devising strategies to mitigate the effects of UV radiation exposure and safeguard cellular health.