Understanding The Polarity Of Water: Partial Charges And Dipole Moment
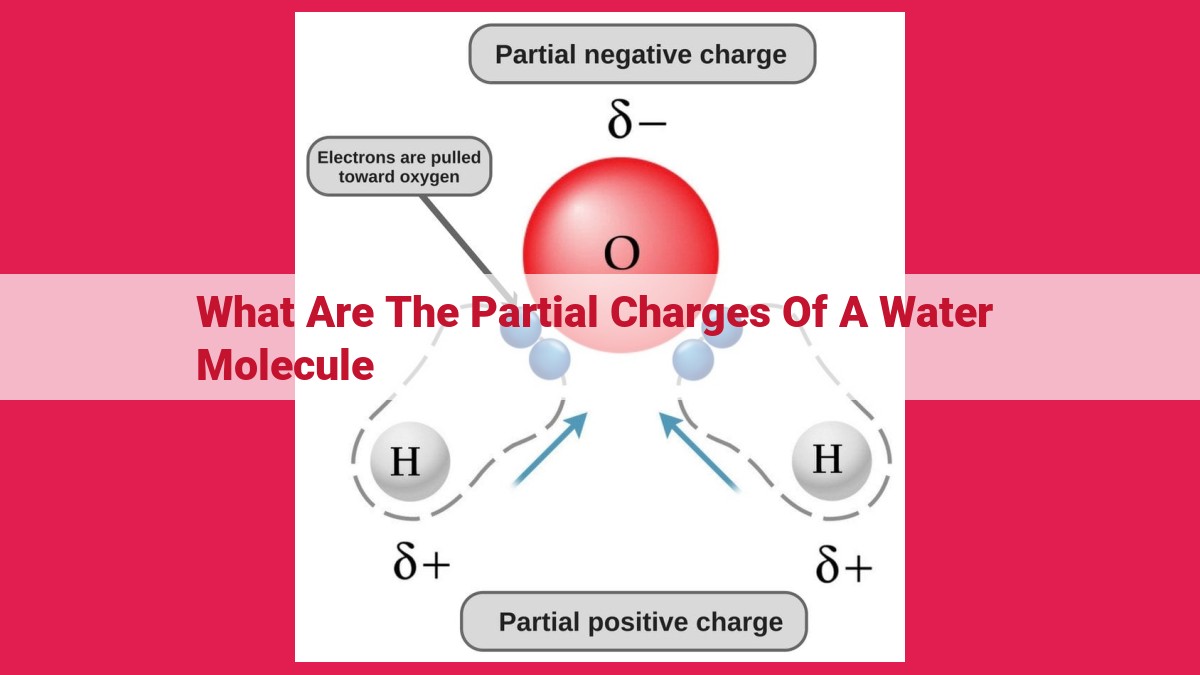
A water molecule exhibits partial charges due to the uneven distribution of electrons between its constituent atoms. Oxygen’s higher electronegativity attracts electrons towards itself, creating a negative partial charge on the oxygen atom. Consequently, the hydrogen atoms develop a positive partial charge due to their lower electronegativity. This charge separation results in the formation of a dipole moment within the water molecule, contributing to its polarity and unique properties.
Partial Charges of a Water Molecule: A Journey into Molecular Polarity
In the world of molecules, understanding the subtle interplay of atoms and electrons is crucial to unraveling their properties and behavior. One such fascinating aspect is the concept of partial charges, which play a significant role in shaping the unique characteristics of water.
Electronegativity and Its Impact on Polarity and Partial Charges
Electronegativity, a measure of an atom’s ability to attract electrons, varies across different elements. In a molecule, atoms with higher electronegativity tend to pull electrons towards them, creating a region of negative charge around themselves. Conversely, atoms with lower electronegativity become slightly positive. This unequal distribution of electrons results in the formation of partial charges.
In a water molecule, oxygen stands out with a higher electronegativity compared to hydrogen. This means that oxygen has a stronger grip on the shared electrons, pulling them closer and leaving the hydrogen atoms with a slight deficiency of electrons. Consequently, a negative partial charge develops on the oxygen atom, while the positive partial charges reside on the hydrogen atoms.
Polarity and Partial Charges
The presence of partial charges within a molecule gives rise to a fascinating concept known as polarity. Polarity refers to the separation of positive and negative charges within a molecule, creating a dipole moment. This dipole moment is a measure of the strength and direction of the polarity.
In the case of water, the partial charges on the oxygen and hydrogen atoms create a dipole moment, making water a polar molecule. This polarity is responsible for the unique properties that distinguish water from other molecules.
Bent Molecular Shape and Partial Charges
Water’s molecular shape is not linear, but rather bent. This bent shape is a direct consequence of the partial charges and the repulsive forces between the electron pairs around the oxygen atom. The lone pairs of electrons on oxygen push away from each other, forcing the hydrogen atoms and their partial positive charges slightly away from each other, resulting in a bent molecular geometry.
The Significance of Water’s Partial Charges
The partial charges of a water molecule play a vital role in its behavior and interactions with other molecules. Water’s polarity allows it to form hydrogen bonds, which are crucial for many biological processes. These hydrogen bonds contribute to the stability and structure of biomolecules like proteins and DNA.
Furthermore, the polarity of water influences its interaction with ions and other polar molecules, making it an excellent solvent for various substances. This property is essential for life as we know it, as water serves as the primary medium for chemical reactions within living organisms.
Electronegativity and Partial Charges
- Explain the electronegativity of oxygen and hydrogen atoms in water.
- Describe how unequal electron sharing results in partial charges.
- Illustrate the electronegativity differences within a water molecule.
Electronegativity and Partial Charges: Unraveling the Unequal Sharing in Water Molecules
In the world of atoms, electronegativity reigns supreme, determining how strongly they attract the electrons that dance around their nuclei. In a water molecule, the story of electronegativity becomes particularly captivating.
Let’s meet the players: the oxygen atom, with its towering electronegativity, and the two hydrogen atoms, their electronegativity a tad lower. As these atoms cozy up, they form covalent bonds, but not just any ordinary bonds. The oxygen atom, with its superior electron-grabbing prowess, pulls the electrons closer to itself, resulting in an unequal sharing of the electron cloud.
This lopsided electron distribution creates partial charges within the water molecule. The oxygen atom, having snagged more electrons, earns a negative partial charge, denoted as δ-. On the flip side, the hydrogen atoms, with their smaller share of the electron pie, are left with positive partial charges (δ+).
Imagine a tug-of-war game where the oxygen atom is the powerhouse and the hydrogen atoms are the underdogs. The stronger oxygen atom pulls the electrons towards its corner, while the hydrogen atoms hang on for dear life, resulting in a tug-of-war that never quite reaches equilibrium.
Polarity and Partial Charges: Unveiling Water’s Unique Structure
In the microscopic world, molecules are not static entities but dynamic entities governed by the interplay of electrical forces. A prime example of this phenomenon is the water molecule, an essential building block of life. Its unique structure and properties stem from the unequal distribution of electrons within the molecule, leading to partial charges.
Partial charges arise when atoms within a molecule have different electronegativities, a measure of their ability to attract electrons. In a water molecule, the oxygen atom is more electronegative than the two hydrogen atoms. This means that the oxygen atom has a greater tendency to pull electrons towards itself, creating a negative partial charge on its side of the molecule. Conversely, the hydrogen atoms have a positive partial charge on their side, as they have a weaker pull on electrons.
This unequal electron distribution results in a polarity within the water molecule. Polarity refers to the separation of positive and negative charges within a molecule. Water is considered a polar molecule because it has a distinct positive and negative end. This polarity is quantified by the dipole moment, a numerical value that measures the strength and direction of the polarity.
The bent shape of a water molecule further enhances its polarity. The tetrahedral geometry of the oxygen atom forces the hydrogen atoms to be positioned at an angle to each other, creating a dipole moment. This dipole moment is responsible for the unique properties of water, including its high surface tension, ability to dissolve many substances, and its role in biological processes.
Bent Molecular Shape and Partial Charges: The Story of Water’s Unique Structure
In the realm of molecules, water stands out with its intriguing bent shape, a characteristic that profoundly influences its behavior. Partial charges, subtle imbalances in electron distribution, play a crucial role in shaping this unique geometry and endowing water with its remarkable properties.
The Electronegativity Dance
Electronegativity is the tendency of atoms to attract electrons towards themselves. In a water molecule, oxygen reigns supreme with its higher electronegativity, while hydrogen atoms play the role of reluctant electron donors. This disparity in electronegativity leads to an unequal sharing of electrons, resulting in partial charges.
The Bent Shape Emerges
The partial negative charge on the oxygen atom and the partial positive charges on the hydrogen atoms create a dipole moment, a vector representing the imbalance of charge within the molecule. This dipole moment pulls the molecule into a bent shape, with the hydrogen atoms oriented away from the oxygen atom.
The Dipole Moment’s Influence
The bent molecular shape has profound implications for water’s behavior. The dipole moment allows water molecules to interact with other polar molecules or surfaces, forming hydrogen bonds. These bonds are essential for a wide range of biological processes, such as the stabilization of DNA and the transport of nutrients in cells.
Water’s Versatility
The polarity of water also contributes to its high surface tension and high boiling point. These properties enable water to form droplets, lubricate surfaces, and resist evaporation, making it an indispensable solvent for countless chemical reactions and biological processes.
Partial charges are the unsung heroes behind water’s bent molecular shape and remarkable properties. Their subtle influence shapes the molecule’s geometry, dipole moment, and interactions with its surroundings. Understanding these partial charges deepens our appreciation for the complexity and elegance of nature’s building blocks.
Partial Charges of a Water Molecule: Unveiling the Story of Dipole Moments and Molecular Shape
Water, the elixir of life, holds secrets that go beyond its simplistic chemical formula, H2O. At its core lies a fascinating tale of partial charges, a concept that unveils the intricacies of its molecular properties and interactions.
Electronegativity, a measure of an atom’s attraction for shared electrons, plays a pivotal role in shaping these partial charges. Oxygen, with its higher electronegativity, exerts a stronger pull on electrons compared to hydrogen. As a result, electrons are unequally distributed, creating a net negative partial charge on the oxygen atom and net positive partial charges on the hydrogen atoms.
These partial charges have profound implications on water’s polarity. Polarity measures the uneven distribution of charge within a molecule, creating a dipole moment. Think of it as a tiny magnet with two opposite poles—a positive end (near the hydrogen atoms) and a negative end (near the oxygen atom). Water’s bent molecular shape, with its hydrogen atoms slightly off-center, further amplifies this dipole moment.
This polar nature of water is not merely an abstract concept; it’s at the heart of myriad biological processes and physical phenomena. From its role as a universal solvent to its significance in intermolecular forces, understanding water’s partial charges provides a window into the intricate tapestry of life and matter.
So, the next time you sip on a glass of water, remember the dance of partial charges. It’s these subtle yet profound differences that赋予 water its unique properties and make it the lifeblood of our planet.
Partial Charges of a Water Molecule: A Story of Electronegativity, Polarity, and Interactions
Water, the elixir of life, is an essential component of all living organisms. Its unique properties, such as its polarity, play a crucial role in many biological processes. To understand these properties, we need to delve into the concept of partial charges within a water molecule.
Electronegativity and Partial Charges
Electronegativity is a measure of an atom’s ability to attract electrons towards itself. In a water molecule, the oxygen atom is more electronegative than the hydrogen atoms. This means that oxygen has a stronger pull on shared electrons, resulting in a slight imbalance in electron distribution. Consequently, the oxygen atom acquires a negative partial charge, while the hydrogen atoms have positive partial charges.
Polarity and Partial Charges
The unequal distribution of charges creates a polarity within the water molecule. The oxygen side becomes slightly negative, while the hydrogen side becomes slightly positive. This polarity is represented by a dipole moment, a measure of the separation of positive and negative charges.
Bent Molecular Shape and Partial Charges
The polarity of water molecules influences their molecular shape. The hydrogen atoms are repelled by each other due to their positive partial charges, resulting in a bent molecular shape. This shape further enhances the dipole moment, making the water molecule even more polar.
Examples and Applications
Biological Processes: Water’s polarity is essential for many biological processes. For example, in cell membranes, the polar water molecules interact with the polar heads of lipid molecules, forming a protective barrier.
Intermolecular Forces: Partial charges also play a role in the interactions between water molecules. The opposite partial charges of different water molecules allow them to form hydrogen bonds, weak electrostatic interactions that influence many properties of water, such as its high boiling point.
Partial charges in a water molecule are a result of the electronegativity differences between oxygen and hydrogen atoms. These partial charges create polarity and a bent molecular shape, which influence water’s interactions and properties. Understanding partial charges is crucial for comprehending the behavior of water in both biological and physical systems.