The Ph Scale: Understanding Neutrality And Its Importance
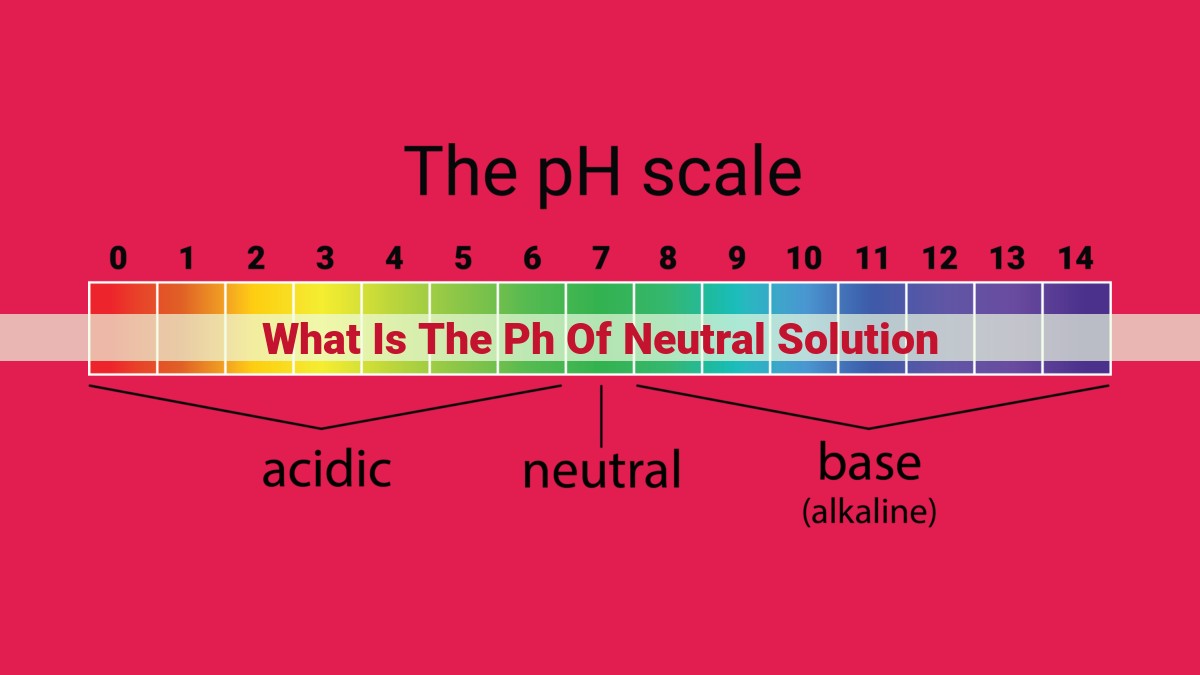
A neutral solution has a pH of 7, indicating equal concentrations of hydrogen and hydroxide ions. This balance makes neutral solutions neither acidic nor basic, crucial for chemical reactions and biological processes. The pH scale measures acidity and alkalinity, with values below 7 representing acids and those above 7 indicating bases. Understanding pH is essential in fields like chemistry, biology, and environmental science.
Understanding the Essence of pH: A Cornerstone of Chemistry and Beyond
In the realm of chemistry, the concept of pH holds immense significance as a measure of a substance’s acidity or alkalinity. pH, which stands for “power of hydrogen,” is a numerical value that quantifies the concentration of hydrogen ions (H+) in a solution. This seemingly simple measurement plays a crucial role in diverse fields, including chemistry, biology, and environmental science, shaping the world around us.
pH is like a chemical compass, guiding us through the intricacies of chemical reactions and biological processes. In chemistry, understanding pH is essential for predicting the behavior of acids and bases. Acids, substances that release hydrogen ions when dissolved in water, have pH values below 7. Conversely, bases, which release hydroxide ions, have pH values above 7. This delicate balance between acids and bases dictates the reactivity of chemicals and governs numerous industrial processes.
Beyond chemistry, pH also influences the health of living organisms and the integrity of our planet. In biology, pH regulates enzyme activity, which is crucial for cellular functions. A slight shift in pH can profoundly impact the growth, metabolism, and reproduction of plants and animals. In environmental science, pH plays a vital role in monitoring water quality, soil health, and the flourishing of ecosystems. Understanding pH empowers us to safeguard our environment and ensure the well-being of all living things.
Concept of pH: A Deeper Dive
- Define pH as the concentration of hydrogen ions in a solution.
- Introduce the pH scale (0-14) and relate it to acidity (pH<7) and alkalinity (pH>7).
- Provide examples of practical applications of pH measurement, such as soil testing and water quality monitoring.
Concept of pH: A Deeper Dive
pH, a measure of acidity or alkalinity, is crucial in various scientific fields, including chemistry, biology, and environmental science. It’s defined as the concentration of hydrogen ions (H+) in a solution.
The pH scale, ranging from 0 to 14, indicates the acidity or alkalinity of a substance. A neutral solution has a pH of 7, indicating equal concentrations of H+ and hydroxide ions (OH-). Acids, like hydrochloric acid (HCl), have pH values below 7, releasing H+ ions into solutions. Bases, such as sodium hydroxide (NaOH), release OH- ions, resulting in pH values above 7.
Understanding pH has numerous practical applications. In soil testing, optimal pH levels are crucial for plant growth and nutrient absorption. In water quality monitoring, pH measurements assess water contamination and its safety for drinking and aquatic life.
By delving into the concept of pH, we gain a deeper appreciation for its significance in scientific research and everyday applications. Understanding the role of acids and bases in determining pH empowers us to make informed decisions about the chemical environment around us.
Neutral Solution: Characteristics and Importance
- Define a neutral solution as one with a pH of 7.
- Explain that neutral solutions have equal concentrations of hydrogen ions and hydroxide ions.
- Discuss the significance of neutral conditions for chemical reactions and biological processes.
Neutral Solutions: The Delicate Balance of Acidity and Alkalinity
In the realm of chemistry, understanding the concept of pH is paramount. It’s a measure that tells us how acidic or alkaline a substance is. Neutral solutions, with a pH of exactly 7, hold a unique position on this scale, balancing the opposing forces of acidity and alkalinity.
Defining Neutrality
A neutral solution is one in which the concentration of hydrogen ions (H+) is equal to the concentration of hydroxide ions (OH-). This delicate balance results in a pH of 7. It’s neither acidic (pH < 7), nor alkaline (pH > 7).
Significance of Neutral pH
Maintaining a neutral pH is crucial for many chemical reactions and biological processes. In these environments, molecules can interact optimally, ensuring proper functioning. For example, in human blood, a pH close to 7 is essential for transporting oxygen and maintaining cell viability.
Understanding the Balance
In neutral solutions, the concentrations of hydrogen ions and hydroxide ions are equal. This means that the solution has neither the capacity to donate nor accept protons. As a result, neutral solutions do not react with strong acids or bases. They simply dilute them.
Practical Applications
Neutral solutions find numerous applications in various fields:
- Water quality: Natural water sources should have a pH close to 7. Deviations from neutrality can indicate contamination.
- Soil health: Soil acidity can affect plant growth. Neutralizing acidic soils with lime can improve crop yields.
- Chemical manufacturing: Many industrial processes require neutral conditions to prevent unwanted reactions.
Neutral solutions play a crucial role in our world, providing a balanced environment for life and chemical reactions to thrive. Understanding their characteristics and importance deepens our knowledge of chemistry and its applications.
Navigating the pH Scale: Understanding the Acidity and Alkalinity Spectrum
The pH scale is a crucial tool in chemistry and various other disciplines, but what exactly is it and how do we interpret it?
The pH scale is a logarithmic scale that measures the concentration of hydrogen ions (H+) in a solution. It ranges from 0 to 14, with each whole number unit representing a tenfold change in hydrogen ion concentration.
0-6.9: Acidic Solutions
Solutions with a pH below 7 are considered acidic. The lower the number on the pH scale, the higher the concentration of hydrogen ions and the more acidic the solution. Common examples of acids are lemon juice (pH 2.2), stomach acid (pH 1.2), and vinegar (pH 2.4).
7.0: Neutral Solutions
A solution with a pH of 7 is neutral, meaning it has an equal concentration of hydrogen ions and hydroxide ions (OH-). Neutral solutions are neither acidic nor basic, and water is a prime example. The pH of pure water is exactly 7.
7.1-14: Alkaline (or Basic) Solutions
Solutions with a pH above 7 are considered alkaline, also known as basic. The higher the number on the pH scale, the lower the concentration of hydrogen ions and the more alkaline the solution. Familiar examples of bases include baking soda (pH 8.3), ammonia (pH 11.6), and household bleach (pH 12.5).
Understanding the pH scale is essential for numerous applications, from soil testing to water quality monitoring. It provides a clear and concise way to assess the acidity or alkalinity of a solution and is a valuable tool for scientific research and everyday life.
Acids and Bases: Unraveling Their Influence on pH
Acids and bases are chemical entities that play a crucial role in shaping the pH of solutions, a measure of their acidity or alkalinity. Understanding the nature and behavior of these substances is essential for grasping the intricacies of pH and its myriad applications.
Acids: The Hydrogen Ion Contributors
Acids are characterized by their ability to release hydrogen ions (H+) when dissolved in water. This release results in an increase in the concentration of H+ ions, leading to a decrease in pH. Common examples of acids include hydrochloric acid(HCl), found in stomach acid, and acetic acid(CH3COOH), the main component of vinegar.
Bases: The Hydroxide Ion Contributors
Bases, on the other hand, are substances that release hydroxide ions (OH-) when dissolved in water. This release causes an increase in pH. Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are familiar examples of bases widely used in cleaning products and industrial processes.
Practical Applications of Acids and Bases
Acids and bases find numerous practical applications in various fields:
- Industry: Acids are used in metalworking, electroplating, and chemical manufacturing. Bases are employed in soap and detergent production, papermaking, and water treatment.
- Agriculture: Acids help adjust soil pH for optimal plant growth. Bases neutralize acidic soils and provide essential nutrients.
- Medicine: Acids are used in the production of aspirin and other pharmaceuticals. Bases are used in antacids and certain topical treatments.
The Dance of Acids and Bases in pH
The interplay between acids and bases is pivotal in determining the pH of a solution. When an acid is added to a base, a neutralization reaction occurs, resulting in the formation of a salt and water. This reaction effectively reduces the concentration of both H+ and OH- ions, leading to a shift towards a neutral pH of 7.
Conversely, when a base is added to an acid, the same neutralization reaction takes place, again resulting in a shift towards neutrality. Understanding the behavior of acids and bases is crucial for controlling the pH of solutions in a wide range of applications, from laboratory experiments to industrial processes.
The pH of Neutral Solutions: Finding Harmony in the Balance
In the vast tapestry of chemistry, the concept of pH holds immense significance, illuminating the acidity or alkalinity of a substance. A neutral solution, like a harmonious symphony, strikes a perfect balance between these opposing forces, maintaining an equilibrium that is crucial for countless chemical reactions and biological processes.
Understanding Neutral Solutions
A neutral solution is characterized by a pH value of exactly 7. This peculiar number represents a delicate balance, where the concentration of hydrogen ions (H+) is precisely equal to the concentration of hydroxide ions (OH-). This equilibrium creates a harmonious state where neither acidity nor alkalinity prevails.
The Magic of 7: Striking a Balance
The pH scale, ranging from 0 to 14, serves as a guide to the acidity or basicity of a solution. Acidic solutions fall below pH 7, while alkaline solutions soar above this midpoint. Neutral solutions, with their pH of 7, occupy the perfect middle ground, showcasing a neutral disposition.
Implications for Everyday Life
Neutral solutions play a pivotal role in our daily lives. The human body, a marvel of biological intricacies, thrives in a near-neutral pH environment. Blood, the life-giving fluid, maintains a pH of around 7.4, allowing for optimal enzyme function and cellular processes.
Applications in Science and Industry
Beyond the human body, neutral solutions find wide-ranging applications in science and industry. In agriculture, soil testing determines acidity levels to guide fertilizer recommendations. In environmental monitoring, neutral solutions serve as benchmarks for assessing water quality. From drug development to food preservation, neutral solutions enable countless technological advancements.
Understanding the pH of neutral solutions unlocks a deeper appreciation for the delicate balance that governs our world. Whether it’s maintaining the harmony of the human body or facilitating scientific breakthroughs, neutral solutions stand as a testament to the power of equilibrium. By embracing the knowledge of pH, we gain insights into the intricate workings of chemistry and the remarkable interplay between acidity and alkalinity.