Understanding Ph Probe Functionality: A Guide To Ph Measurement
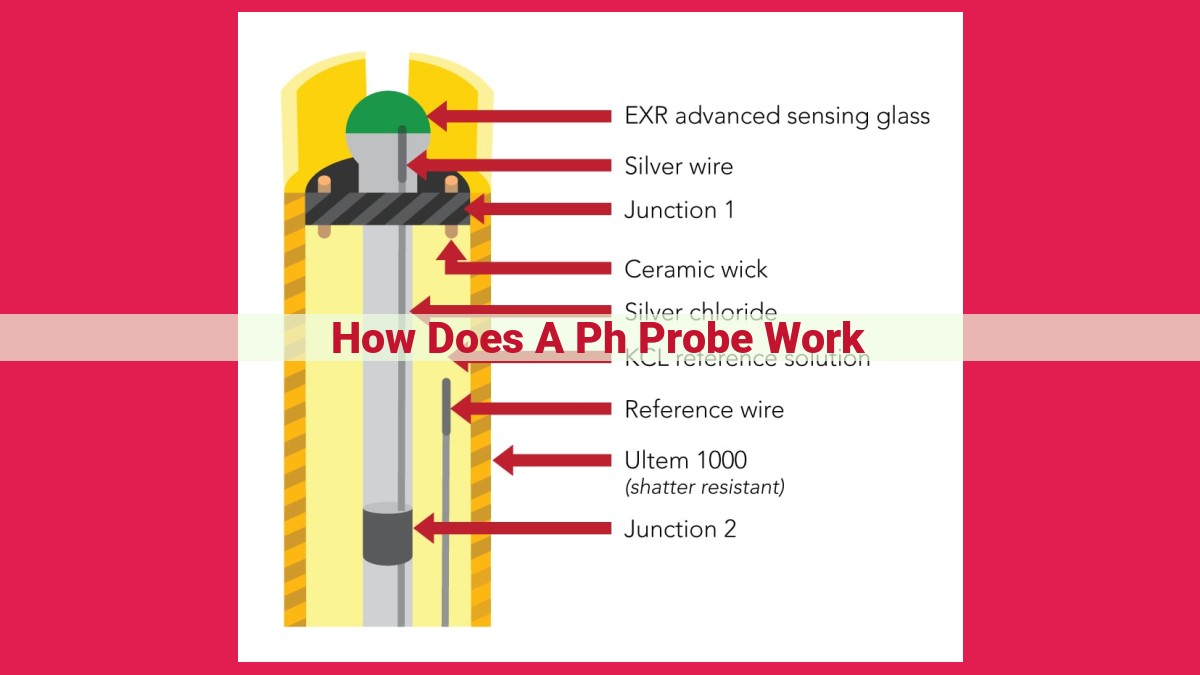
How Does a pH Probe Work?
pH probes measure pH, a measure of acidity or alkalinity, by detecting the potential difference between a glass electrode and a reference electrode. The glass electrode has a hydrogen ion-selective membrane that allows hydrogen ions to pass through, generating a potential difference that varies with hydrogen ion concentration. The reference electrode maintains a constant potential, allowing a potentiometer to measure the difference in potential between the two electrodes, which is directly proportional to pH.
The Magic of Measuring pH: Unveiling the Secret of pH Probes
In the realm of chemistry and beyond, there exists a hidden yet influential parameter that governs the behavior of substances: pH. Embark on a captivating journey to unravel the enigma of pH and its pivotal role in shaping our world.
Defining pH: The Essence of Acidity and Alkalinity
Imagine a magical scale that can quantify the acidity or alkalinity of a substance. This magical scale, known as the pH scale, ranges from 0 to 14. At the neutral point of 7, the substance is neither acidic nor alkaline (also known as basic). Below 7, the substance is increasingly acidic, while above 7, it is alkaline.
The Role of pH Probes: Unveiling the Hidden Truth
Just as detectives use tools to uncover hidden clues, scientists use specialized instruments called pH probes to accurately measure pH levels. These probes rely on the remarkable properties of glass and their ability to sense the presence of hydrogen ions, the tiny particles responsible for acidity.
Inside a pH Probe: A Tale of Two Electrodes
At the heart of every pH probe lies a fascinating dance between two electrodes: the glass electrode and the reference electrode. The glass electrode, with its ion-selective membrane, acts as a sort of gatekeeper, allowing only hydrogen ions to pass through. Its sensitive membrane generates an electrical signal proportional to the concentration of hydrogen ions in the solution.
The reference electrode, on the other hand, provides a stable potential against which the glass electrode can be compared. This potential difference, measured by a specialized device called a potentiometer, is directly related to the pH of the solution.
Delving into the Heart of a pH Probe: A Story of Precision
Beneath the seemingly simple exterior of a pH probe lies an intricate symphony of electrochemical phenomena. Two key components orchestrate this symphony: the glass electrode and the reference electrode.
A Tale of Two Electrodes: The Glass and Reference Electrodes
At the heart of the glass electrode beats a hydrogen ion-selective membrane, a gatekeeper that allows hydrogen ions (H+) to pass while repelling other ions. This membrane’s selectivity stems from its unique composition, which mimics the cell membrane of living organisms.
Complementing the glass electrode is the reference electrode, a silent maestro that maintains a stable voltage. Acting as a point of comparison, it interacts with the glass electrode to create a potential difference, the key to unlocking pH measurement.
Unveiling the pH Measurement Riddle
The potential difference between the glass and reference electrodes holds the secret to pH. As the hydrogen ion concentration in the solution changes, so too does the potential difference. This correlation is the basis of pH measurement.
A potentiometer, a voltage-measuring device, steps into the scene. By comparing the potential difference between the glass and reference electrodes to a known voltage, it determines the hydrogen ion concentration. This value, when translated into its logarithmic form, gives us the coveted pH reading.
Empowering Our Understanding: Key Concepts Demystified
- Ion-Selective Membrane: The heart of the glass electrode, allowing H+ ions to dance freely while barring others.
- Acidic or Alkaline Solutions: These solutions influence pH measurement, with acidic solutions hosting high H+ concentrations and alkaline solutions dwindling.
- Calibration: Essential for accurate readings, ensuring the probe aligns with known pH standards.
- Temperature Compensation: pH readings fluctuate with temperature; compensation adjusts for these variations, ensuring reliable data.
A Versatile Tool: pH Measurement in Action
pH probes wield their precision across a vast array of industries, transforming our understanding of the world around us.
- Food Industry: Determining food quality, ensuring safety, and maximizing shelf life.
- Water Treatment: Maintaining water purity and optimizing chemical processes.
- Healthcare: Monitoring physiological processes, diagnosing diseases, and guiding treatment.
The intricate interplay of the glass and reference electrodes within a pH probe empowers us with precise pH measurements. These measurements illuminate various aspects of our world, from ensuring the safety of our food and water to advancing medical knowledge. The pH probe, a testament to scientific innovation, continues to unveil the secrets of our environment, one precise measurement at a time.
Unveiling the pH Measurement Principle
Understanding the principles behind pH measurement is like embarking on a scientific expedition. Let’s unravel the mysteries and uncover the secrets of this crucial technique.
At the heart of pH measurement lies the correlation between hydrogen ion concentration and potential difference. Hydrogen ions, those tiny soldiers of acidity, are always on the move, creating an ion gradient. This gradient, like a battlefield, is measured using a voltmeter called a potentiometer.
Imagine the potentiometer as a messenger, translating the ion gradient into an electrical signal. This signal is a measure of the potential difference, a key value in determining pH. The higher the hydrogen ion concentration, the greater the potential difference.
But wait, there’s more to this tale. The potential difference is not directly proportional to pH. Instead, it follows a logarithmic relationship. This means that even small changes in pH can result in significant changes in potential difference.
So, how do we convert this potential difference into a pH value? Enter the pH probe. This clever device uses the relationship between potential difference and pH to determine the acidity or alkalinity of a solution.
In essence, pH measurement is a dance between hydrogen ions, potential difference, and the ever-reliable potentiometer. By understanding this dance, we can unlock the power of pH measurement and gain valuable insights into the chemical world around us.
Key Concepts to Know
- Ion-Selective Membrane: Discuss its crucial role in pH sensitivity.
- Acidic or Alkaline Solutions: Explore their influence on pH measurement.
- Calibration: Emphasize its importance for accurate readings.
- Temperature Compensation: Explain the need to adjust pH readings for temperature variations.
Key Concepts to Unlock the Secrets of pH Measurement
To delve into the intricacies of pH measurement, it’s crucial to grasp a few key concepts that illuminate the underlying principles.
Ion-Selective Membrane: The Gatekeeper of pH Sensitivity
At the heart of a pH probe lies the ion-selective membrane, a semipermeable barrier that responds to hydrogen ions with remarkable sensitivity. This membrane, composed of glass or specific polymers, allows only hydrogen ions to pass through its microscopic pores, selectively屏蔽其他离子.
Acidic and Alkaline Solutions: The pH Spectrum’s Extremes
The pH range is a scale that measures the acidity or alkalinity of a solution. Acidic solutions possess an abundance of hydrogen ions, resulting in lower pH values (below 7). Conversely, alkaline solutions have fewer hydrogen ions, leading to higher pH values (above 7).
Calibration: The Key to Precision
To ensure accurate pH readings, calibration is essential. Calibration involves comparing the probe’s readings to a known pH standard solution and adjusting the instrument accordingly. This process corrects for any drift or deviations in the probe’s performance over time.
Temperature Compensation: Embracing the Influence of Heat
Temperature significantly affects pH readings. As temperature rises, the ionization of water increases, leading to more hydrogen ions and a lower pH. To compensate for this, pH probes incorporate temperature compensation mechanisms that automatically adjust the readings based on the solution’s temperature.
Practical Applications of pH Measurement Across Industries
pH measurement plays a pivotal role in various industries, serving as a fundamental tool for quality control, research, and monitoring.
Food Industry: The food industry relies heavily on pH measurement to ensure the safety, shelf life, and taste of products. In the dairy industry, pH monitors ensure proper acidity levels in milk, cheese, and yogurt. Beverage manufacturers utilize pH meters to control acidity, sweetness, and flavor profiles in products such as beer, wine, and juice.
Water Treatment: pH measurement is crucial in water treatment facilities to optimize chemical processes and ensure the safety of drinking water. Municipalities use pH probes to control pH levels in drinking water sources and wastewater treatment plants to neutralize acidity and remove pollutants.
Healthcare: pH measurement is essential in medical diagnostics and patient care. Physicians use pH test strips to monitor acidity levels in bodily fluids such as urine and saliva. Pharmaceutical companies utilize pH meters in drug development to determine the stability and efficacy of new medications.
Biological Research: pH measurement is an indispensable tool in biological research, providing insights into cellular processes and environmental interactions. Scientists use pH probes to study the pH optima of enzymes, growth rates of microorganisms, and the impact of environmental factors on marine ecosystems.
Agricultural Applications: pH measurement plays a vital role in agriculture, aiding in soil management and crop production. Farmers use pH meters to monitor soil pH levels and determine the appropriate amount of fertilizers and amendments needed for optimal crop yields. Horticulturists measure pH levels in irrigation water to ensure proper nutrient availability for plants.
By understanding the fundamental principles of pH measurement, we can harness its power to enhance our lives, from ensuring the safety of our food and water to advancing medical research and agricultural practices.