Understanding Ph From Molarity: A Comprehensive Guide For Determining Solution Acidity/Alcalinity
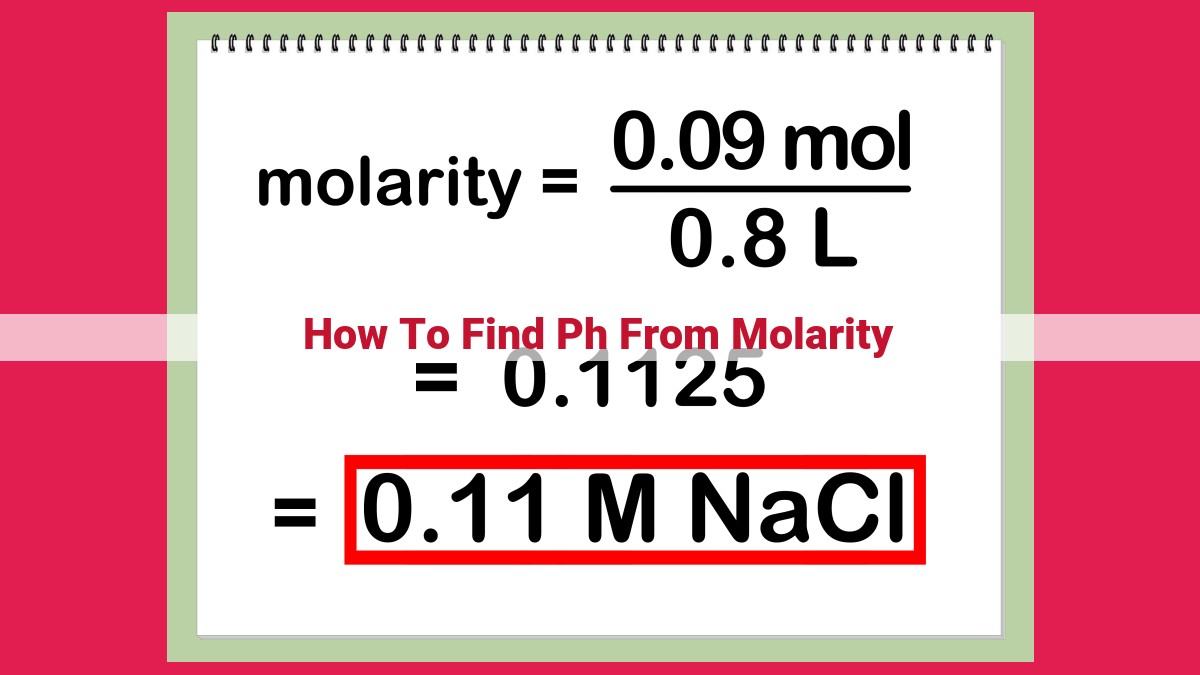
Finding pH from molarity requires understanding the concentration and acidity/alkalinity of solutions. For acidic solutions, convert molarity to [H+] and calculate pH using pH = -log[H+]. For alkaline solutions, convert molarity to [OH-], calculate pOH using pOH = -log[OH-], and calculate pH using pH = 14 – pOH. By understanding these steps and the relationships between molarity, [H+], [OH-], and pH, you can accurately determine the acidity/alkalinity of solutions.
Unveiling the Secrets of pH: A Comprehensive Guide
Embarking on a Journey to Understand pH
The ability to determine the acidity or alkalinity of a substance is crucial in a myriad of fields. From understanding the workings of living organisms to optimizing industrial processes, pH plays a pivotal role. The relationship between pH and molarity, a measure of solution concentration, provides us with a valuable tool to delve into the nature of these substances.
pH stands as a numerical scale that quantifies the acidity or alkalinity of a solution. It is measured on a logarithmic scale ranging from 0 to 14, with values below 7 indicating acidity, above 7 signaling alkalinity, and 7 representing neutrality. Molarity, on the other hand, measures the number of moles of solute present per liter of solution. Establishing the Connection Between pH and Molarity
The connection between pH and molarity stems from the behavior of certain substances in water. When acids dissolve in water, they release hydrogen ions (H+), which contribute to acidity. Conversely, bases release hydroxide ions (OH-), elevating alkalinity. The concentration of these ions in solution directly influences the pH value.
For strong acids and bases, their molarity corresponds directly to the concentration of H+ and OH- ions, respectively. In such cases, determining pH from molarity becomes a straightforward process. However, for weak acids and bases, the relationship is more complex due to their partial ionization in water.
Navigating the pH Calculations
To determine pH from molarity, the following steps are essential:
-
For Acidic Solutions:
- Convert molarity to [H+]: [H+] = Molarity of acid
- Calculate pH: pH = -log[H+]
-
For Alkaline Solutions:
- Convert molarity to [OH-]: [OH-] = Molarity of base
- Calculate pOH: pOH = -log[OH-]
- Calculate pH: pH = 14 – pOH
Concepts
- Define molarity and explain its significance in measuring solution concentration.
- Introduce pH as a measure of acidity or alkalinity.
- Explain pOH as a measure of alkalinity.
- Define the ionization constant of water (Kw).
- Define [H+] and [OH-] and their relationship to acidity and alkalinity.
Concepts: Understanding the Basics
In order to find the pH of a solution, we first need to understand some key concepts. Molarity is a measure of the concentration of a solution, expressed as the number of moles of solute per liter of solution. It’s a convenient way to quantify the amount of a substance present in a solution.
pH is a measure of the acidity or alkalinity of a solution, specifically the concentration of hydrogen ions (H+). It ranges from 0 to 14, with 7 representing a neutral solution. Solutions with a pH below 7 are acidic, while those with a pH above 7 are alkaline or basic.
pOH is a measure of alkalinity, specifically the concentration of hydroxide ions (OH-). It’s related to pH by the equation pH + pOH = 14. Therefore, a solution with a high pOH will have a low pH and vice versa.
The ionization constant of water (Kw) is a constant that describes the equilibrium between water molecules and hydrogen and hydroxide ions. At 25°C, Kw = 1.0 x 10^-14. This means that in pure water, the concentration of H+ ions is equal to the concentration of OH- ions, and the solution is neutral.
[H+] and [OH-] represent the molar concentrations of hydrogen and hydroxide ions in a solution, respectively. Their relative concentrations determine the acidity or alkalinity of the solution. A high [H+] indicates an acidic solution, while a high [OH-] indicates an alkaline solution.
Calculating pH from Molarity: A Step-by-Step Guide
In the realm of chemistry, understanding the acidity or alkalinity of a solution is crucial. This is where pH, a measure of acidity or alkalinity, comes into play. While molarity quantifies the concentration of a solution, it’s also closely intertwined with pH. This guide will unveil the steps to calculate pH from molarity, empowering you to decipher the chemical nature of solutions.
For Acidic Solutions:
Acids release hydrogen ions (H+) when dissolved in water. To determine the pH of an acidic solution, follow these steps:
-
Convert molarity to [H+]. The molarity (M) represents the number of moles of acid per liter of solution. Therefore, to obtain the molar concentration of hydrogen ions ([H+]), simply use the formula:
[H+] = M -
Calculate pH using the formula pH = -log[H+]. The pH scale ranges from 0 to 14, with 7 being neutral. Values below 7 indicate acidity, and the lower the pH, the more acidic the solution. The pH formula harnesses the power of logarithms to convert the hydrogen ion concentration ([H+]) to pH:
pH = -log[H+]
For Alkaline Solutions:
Unlike acids, alkaline solutions release hydroxide ions (OH-) when dissolved in water. To calculate the pH of an alkaline solution, follow these steps:
-
Convert molarity to [OH-]. Similar to acids, the molarity of an alkaline solution represents the moles of base per liter. To determine the molar concentration of hydroxide ions ([OH-]), use the same formula as before:
[OH-] = M -
Calculate pOH using the formula pOH = -log[OH-]. pOH measures the alkalinity of a solution. The formula mirrors the pH formula, using the hydroxide ion concentration ([OH-]):
pOH = -log[OH-] -
Calculate pH using the formula pH = 14 – pOH. Since pH and pOH are inversely proportional, you can use pOH to calculate pH:
pH = 14 - pOH
Examples and Applications:
Mastering pH calculations from molarity opens doors to a wide range of applications:
Chemistry: Determine the pH of acidic or alkaline solutions in chemical reactions.
Biology: Understand the pH requirements for enzymatic reactions and cellular processes.
Environmental Science: Monitor the pH of water bodies and soil to assess environmental impact.
By unraveling the secrets of pH and molarity, you’ll gain a deeper understanding of the chemistry that shapes our world. Remember, if you encounter any challenges, don’t hesitate to explore additional resources or consult experts for further guidance.
Examples and Applications of pH Measurement
Calculating pH from Molarity: Examples
Let’s illustrate with examples to make things clearer. Suppose we have a solution with a molarity of 0.1 M for hydrochloric acid (HCl), a strong acid. Using the formula pH = -log[H+], we can calculate the pH as follows:
pH = -log(0.1) = 1
Another example, this time with a weak base like sodium hydroxide (NaOH). For a 0.01 M NaOH solution:
pOH = -log(0.01) = 2
pH = 14 - pOH = 12
Practical Applications of pH Measurement
pH measurement finds widespread use in various fields:
Chemistry:
- Determining the strength of acids and bases
- Monitoring reactions and optimizing chemical processes
Biology:
- Maintaining pH levels in cells and organisms
- Understanding the behavior of enzymes and proteins
Environmental Science:
- Assessing water quality for human health and ecological balance
- Controlling pH in agricultural soil for optimal plant growth
Examples of Practical Applications:
- In agriculture, soil pH is crucial for crop health. A pH of around 6-7 is ideal for most plants.
- In the food industry, pH control is essential for preserving foods and maintaining their quality and safety.
- In the pharmaceutical industry, pH plays a vital role in drug development and manufacturing.
Understanding pH is crucial for various scientific and practical applications. By grasping the relationship between pH and molarity, we can accurately measure and interpret the acidity or alkalinity of solutions. Whether it’s in the lab or in our daily lives, pH plays a significant role in shaping our world.