Understanding Oxygen’s Reactivity: Valence Electrons And Chemical Bonding
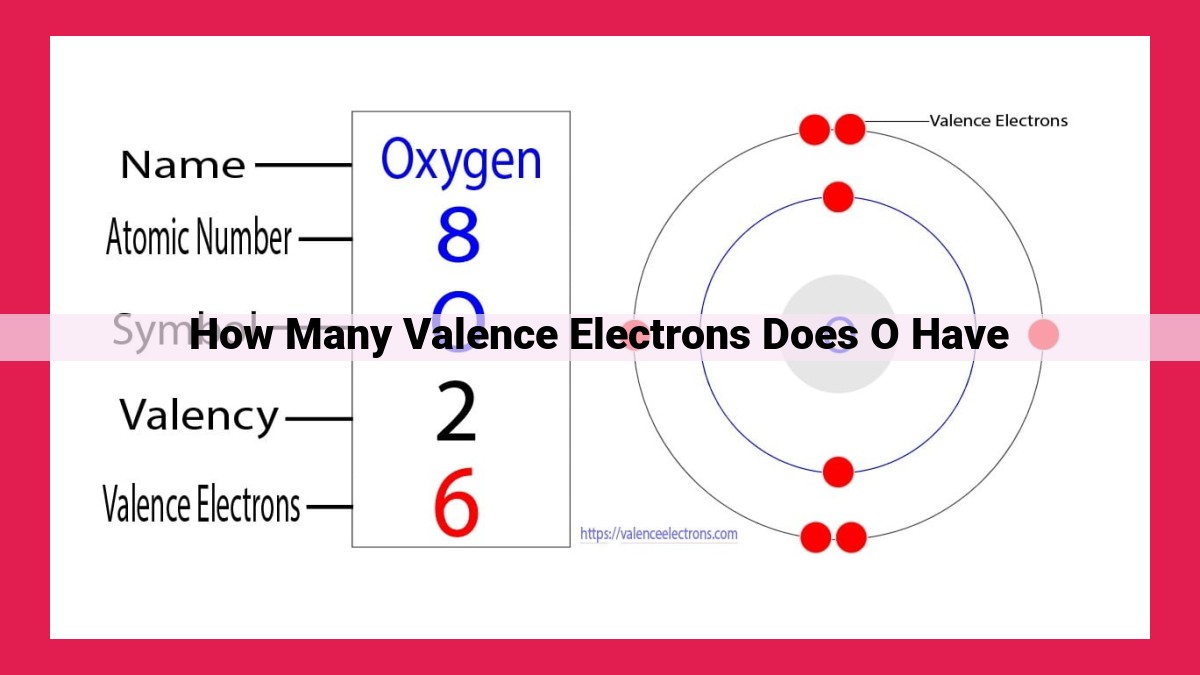
Oxygen, a reactive nonmetal, has 6 valence electrons, which play a crucial role in its chemical bonding. Valence electrons are the outermost electrons in an atom, determining its chemical properties. Oxygen’s high electronegativity and reactivity stem from its valence electrons, enabling it to form stable bonds with other elements. The number and arrangement of valence electrons influence oxygen’s ability to participate in chemical reactions, making it essential for understanding its reactivity and behavior in various chemical processes.
Valence Electrons: The Power Behind Chemical Bonding
In the realm of chemistry, valence electrons reign supreme. These enigmatic electrons, located in the outermost shell of an atom, play a pivotal role in determining an element’s chemical behavior.
Imagine a high-stakes negotiation where atoms seek to form alliances, forging bonds with one another. The stakes are high—the stability of molecules and the very fabric of matter itself hangs in the balance. And it’s the valence electrons, with their unyielding determination, that orchestrate this intricate dance of bonding.
Just as the number of protons within an atom defines its identity, the number of valence electrons it possesses shapes its destiny. These electrons, like tireless diplomats, venture beyond the atom’s inner sanctum to engage in bonding ventures with neighboring atoms.
Their presence or absence, their eagerness to participate in the bonding game, ultimately determines an element’s chemical personality. From the gregarious nature of metals, eager to share their valence electrons and form bonds, to the standoffishness of noble gases, content in their electron solitude, it’s all dictated by the valence electrons’ dance.
Understanding valence electrons is akin to unlocking the secrets of chemistry’s grand ballet. They are the key to comprehending the formation of molecules, the reactivity of elements, and the myriad chemical reactions that shape our world.
Properties of Oxygen
In the realm of chemistry, oxygen stands out as a nonmetallic element, characterized by its lack of metallic luster and poor electrical conductivity. Its unique properties have played a pivotal role in shaping the world we live in.
Electronegativity, a measure of an atom’s ability to attract electrons, is a defining trait of oxygen. With a high electronegativity, oxygen atoms possess a strong pull towards electrons, making them likely to form ionic bonds with elements possessing lower electronegativities. This characteristic enables oxygen to readily form compounds with metals, creating oxides like iron oxide (rust).
Furthermore, oxygen’s reactivity is a testament to its eagerness to participate in chemical reactions. Its valence electrons, responsible for chemical bonding, confer upon oxygen a pronounced affinity for electrons. This reactivity manifests itself in oxygen’s ability to form bonds with a wide array of elements, including hydrogen, carbon, and nitrogen, resulting in the formation of essential molecules like water, carbon dioxide, and nitric oxide.
Valence Electrons of Oxygen: Unlocking the Key to Its Reactivity
In the realm of chemistry, valence electrons are like the social butterflies of atoms, responsible for their ability to connect and react with each other. For oxygen, an element essential to all life forms, its valence electrons play a crucial role in shaping its unique properties and high reactivity.
Oxygen, the third most abundant element in the universe, belongs to the nonmetallic group. Its atoms are characterized by their high electronegativity, meaning they have a strong affinity for electrons. This electronegative nature, coupled with oxygen’s relatively small atomic size, explains its tendency to form chemical bonds with almost every other element.
To understand oxygen’s reactivity, we need to delve into its electronic configuration. Each oxygen atom has eight electrons distributed across its energy levels, with six of them occupying the outermost shell. These six valence electrons are the gateway to oxygen’s chemical versatility.
With its valence electrons eager to join the party, oxygen is highly reactive. It readily forms bonds with other elements by sharing or gaining electrons to achieve a stable configuration of eight valence electrons. This quest for stability drives oxygen’s participation in a wide range of chemical reactions, from combustion to respiration.
In summary, oxygen’s valence electrons are the key to unlocking its reactivity. Their high number and the atom’s electronegative nature enable oxygen to form bonds and participate in chemical reactions, shaping its essential role in our world.
Oxygen’s Reactivity: A Tale of Valence Electrons
Oxygen stands as a chemical maestro, orchestrating a myriad of reactions that shape our world. Its extraordinary reactivity stems from a unique dance performed by its valence electrons. These electrons, the outermost denizens of oxygen’s atomic orchestra, possess an unquenchable thirst for interaction.
In the realm of bonding, valence electrons are the architects of change. Oxygen’s six valence electrons grant it the power to form bonds with a vast array of elements, forging molecules that orchestrate life’s very symphony. This electron-sharing ability propels oxygen into the heart of chemical reactions, where it emerges as a catalyst for transformation.
One striking testament to oxygen’s reactivity lies in its eagerness to oxidize. When oxygen encounters other substances, its valence electrons leap at the chance to snatch electrons from them, creating new compounds that alter the very nature of matter. This oxidizing prowess underlies processes as varied as the rusting of metals and the combustion of fuels, driving the engines of industry and powering the warmth of our homes.
Example: When iron atoms come into contact with oxygen, their valence electrons succumb to oxygen’s allure, forming bonds that give rise to iron oxide, the familiar reddish substance we know as rust. Similarly, in the combustion of wood, oxygen’s insatiable appetite for electrons ignites the chemical bonds holding the wood together, releasing energy as flames dance and devour.
In conclusion, oxygen’s valence electrons stand as the unsung heroes behind its unparalleled reactivity. By embracing their electron-sharing nature, these valence electrons enable oxygen to forge bonds, oxidize substances, and power countless chemical reactions that shape our world from the microscopic to the grandest of scales.
Significance of Valence Electrons in Oxygen’s Reactivity
Understanding valence electrons is crucial for comprehending oxygen’s exceptional chemical reactivity. Valence electrons are the outermost electrons in an atom that participate in chemical bonding. In oxygen’s case, it possesses six valence electrons.
These valence electrons empower oxygen to form chemical bonds with a wide range of elements. When interacting with other elements, oxygen’s valence electrons eagerly seek to complete their electron shells, achieving a stable electron configuration. This quest for stability drives oxygen’s high reactivity and its ability to participate in countless chemical reactions.
Oxygen’s valence electrons enable it to form single, double, or even triple bonds, depending on the element it encounters. For instance, in water (H2O), oxygen forms single bonds with two hydrogen atoms. In carbon dioxide (CO2), it engages in double bonds with carbon. Moreover, in ozone (O3), oxygen exhibits triple bonds with its fellow oxygen atoms.
The versatility of oxygen’s valence electrons extends beyond simple bond formation. It also allows oxygen to participate in oxidation-reduction reactions, where it gains or loses electrons to achieve a stable configuration. This remarkable versatility makes oxygen a vital participant in biological processes, industrial applications, and various environmental phenomena.
In summary, oxygen’s valence electrons play a pivotal role in its chemical reactivity. These outermost electrons drive oxygen’s ability to form bonds with other elements, engage in oxidation-reduction reactions, and contribute to a myriad of chemical processes that shape our world.