Understanding Oxygen: Atomic Number, Protons, And Nuclear Charge Explained
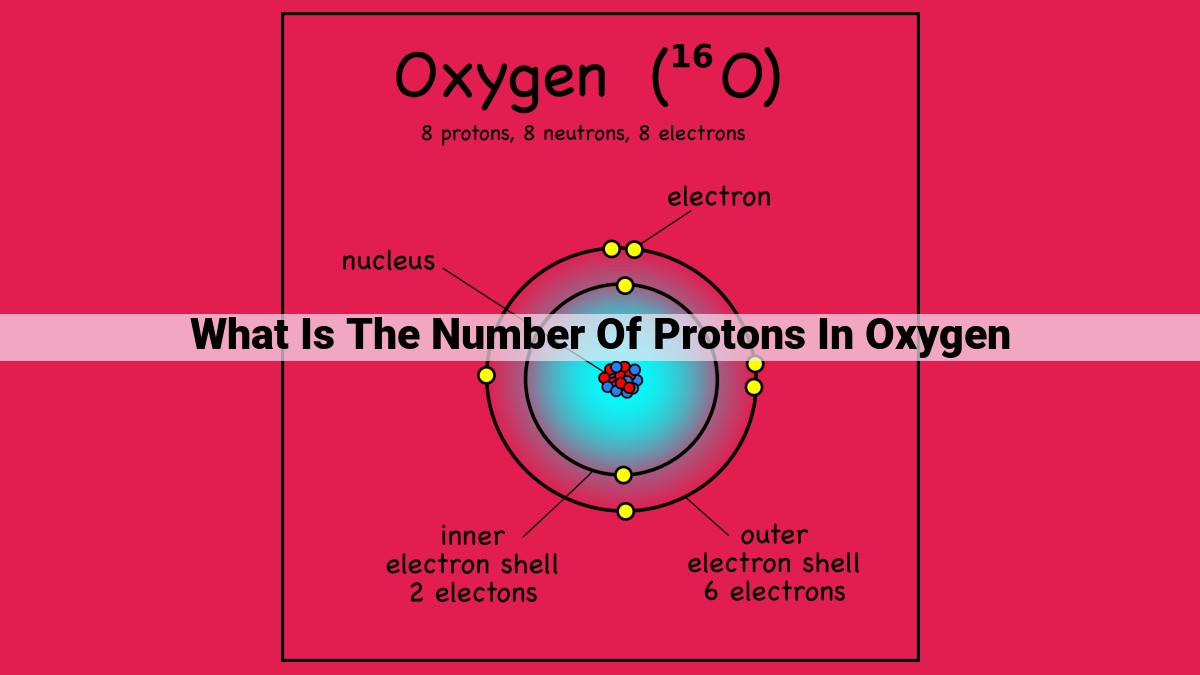
Oxygen, with an atomic number of 8, possesses 8 protons in its nucleus. The number of protons determines an element’s identity and defines its nuclear charge, which in oxygen’s case is +8. Protons reside in the dense core of the atom, balancing the negative charges of electrons to maintain a neutral overall charge.
Atomic Number: Unveiling the Identity of Elements
In the vast realm of chemistry, understanding the fundamental nature of elements is crucial. Atomic number, a pivotal concept, plays a central role in distinguishing one element from another. It’s like the unique fingerprint that identifies each element, unlocking its identity and characteristics.
What is Atomic Number?
In the heart of every atom lies its nucleus, a dense and positively charged region. Within this bustling nucleus reside protons, fundamental particles that carry a positive electric charge. The atomic number of an element is simply the number of protons found within its nucleus.
Consider oxygen, a vital component of our atmosphere. Oxygen’s atomic number is 8, indicating that it possesses 8 protons in its nucleus. This specific number sets oxygen apart from all other elements.
The Significance of Atomic Number
The atomic number of an element is not just a mere number; it holds immense significance in determining the element’s properties and behavior. It governs the nuclear charge, the sum of positive charges from protons within the nucleus. In oxygen’s case, its nuclear charge is +8, which influences its chemical reactivity and interactions with other molecules.
Furthermore, the atomic number directly correlates with the number of electrons in a neutral atom. Electrons, negatively charged particles that balance the positive charge of protons, are present in equal numbers to protons in a neutral atom. This harmonious balance ensures that overall, the atom maintains a neutral electrical charge.
Protons: The Core of the Atom
Protons reside in the nucleus of the atom, alongside neutrons, particles with no electric charge. The number of protons in the nucleus remains constant for a given element, providing a reliable means of identifying it. The atomic number serves as a constant identifier, defining the fundamental characteristics that distinguish one element from another.
Atomic number is an indispensable concept in chemistry, providing the cornerstone of our understanding of elements. It not only identifies elements but also dictates their nuclear charge and drives their chemical behavior. By comprehending the significance of atomic number, we delve deeper into the captivating world of chemistry, unraveling the intricate tapestry of matter that shapes our universe.
Nuclear Charge: A Measure of Proton Power
Just like each of us has a unique signature that sets us apart, every element in the periodic table possesses a distinct identity. This identity is determined by its atomic number, which tells us the number of protons nestled within the heart of its atoms.
Imagine the nucleus of an atom as a bustling metropolis, where positively charged protons and neutral neutrons form the bustling population. The nuclear charge, the collective positive charge of these protons, serves as a measure of the nucleus’s electrical prowess. It’s like the total voltage that powers this atomic city.
Take oxygen, for instance, a life-giving element crucial for our existence. Its atomic number is 8, revealing that each oxygen atom boasts 8 protons. These protons, like tiny powerhouses, contribute a total positive charge of +8 to the oxygen nucleus. This nuclear charge is the driving force behind oxygen’s chemical behavior, influencing its interactions with other elements and shaping its properties.
Protons in the Nucleus: The Core of the Atom
At the very heart of every atom, invisible to the naked eye, lies a tiny but mighty realm called the nucleus. This dense and compact region houses some of the most fundamental building blocks of matter: protons and neutrons.
Protons: The Gatekeepers of Identity
Protons are positively charged subatomic particles that reside within the nucleus. They play a pivotal role in determining the unique characteristics of each element. The number of protons in an atom, known as the atomic number, is its identity card.
For instance, oxygen, an essential element for life, boasts an atomic number of 8. This means that every oxygen atom contains exactly 8 protons in its nucleus. This number distinguishes oxygen from all other elements, making it the building block of oxygen molecules and a crucial component of our atmosphere.
The Dance of Protons and Neutrons
Protons share a cozy neighborhood with their equally important companions, neutrons. Neutrons, as their name suggests, carry no electrical charge. Together, protons and neutrons form the atomic nucleus, a densely packed powerhouse at the center of the atom.
The dance between protons and neutrons is delicate yet vital for the atom’s stability. Their combined presence creates a balance of forces that holds the nucleus together, preventing the atom from collapsing under its own gravity.
Delving into the Heart of Matter: Atomic Number, Nuclear Charge, and the Balancing Act of Electrons
As we embark on a journey into the intricate realm of the atom, we will uncover the fundamental concepts that define the very essence of matter. Join us as we demystify atomic number, nuclear charge, protons, and the delicate balance of electrons that determines an atom’s neutrality.
Atomic Number: The Fingerprint of an Element
Every element has a unique identity, a fingerprint encoded within its atomic number. This number represents the number of protons residing in the atom’s nucleus, the dense core that harbors its positive charge. For instance, oxygen, a vital component of life, boasts an atomic number of 8, indicating the presence of eight protons within its nucleus.
Nuclear Charge: A Reflection of Proton Power
The nuclear charge, another crucial aspect of an atom, measures the collective positive charge emanating from its protons. Just as protons define an element’s identity, their combined charge shapes its nuclear character. In the case of oxygen, with its eight protons, the nuclear charge is an impressive +8.
Protons in the Nucleus: The Atom’s Unwavering Core
Nestled within the atom’s heart, the nucleus is a bustling hub where protons and neutrons coexist. The number of protons within the nucleus determines the atomic number. Oxygen, with its eight protons, proudly carries this atomic insignia within its nuclear fortress.
Electron Count in a Neutral Atom: Seeking Equilibrium
In the realm of atoms, a delicate balance exists between protons and electrons. In a neutral atom, this balance is maintained when the number of electrons equals the number of protons. Oxygen, with its eight protons, accommodates eight electrons to achieve this harmonious state.
This dance of charges ensures that the atom’s overall charge remains neutral, neither positive nor negative. By understanding this intricate interplay of atomic number, nuclear charge, and electron count, we gain deeper insight into the fundamental building blocks of the universe we inhabit.