Unlocking The Power Of Oxidation Numbers: Understanding Nitrogen’s Electronic Structure
Oxidation numbers represent the hypothetical charge of nitrogen atoms in a chemical compound. They help predict the electronic structure and electronegativity of nitrogen. In neutral nitrogen atoms, the oxidation number is zero. In ionic compounds involving nitrogen, the oxidation number typically reflects the charge of the nitrogen-containing ion, such as nitrate (+5), nitrite (+3), ammonium (-3), and ammonia (-3). Oxidation numbers not only aid in predicting chemical reactions but also provide insights into the electronic configuration, bond formation, and molecular polarity of nitrogen compounds.
- Explain what oxidation numbers are and their significance in chemistry.
Oxidation Numbers: Unraveling the Language of Chemical Reactions
In the fascinating world of chemistry, understanding the language of atoms and molecules is essential. Among the most fundamental concepts is the oxidation number, a magical tool that helps us decipher the electronic interactions within chemical compounds. It tells us who’s giving, who’s receiving, and who’s playing fair when electrons dance around the molecular stage.
What’s an Oxidation Number?
Think of an oxidation number as an imaginary charge assigned to an atom within a compound. It represents how many electrons that atom has lost or gained, based on the assumption that all bonds are ionic. This number can be positive, negative, or even zero, depending on the element and its environment.
Significance of Oxidation Numbers
Oxidation numbers are like a backstage pass to the secretive world of chemical reactions. They give us a glimpse into the motivations and behaviors of atoms, allowing us to predict how they will interact with each other. By studying oxidation numbers, we can unravel the mystery behind what drives chemical reactions, particularly those involving the transfer of electrons: the famous redox reactions.
Oxidation Number of Nitrogen: Unveiling the Reactivity of a Versatile Element
Nitrogen, the seventh element on the periodic table, is a fascinating and multifaceted element that plays a crucial role in countless chemical reactions. Understanding its oxidation number, a fundamental concept in chemistry, is essential for deciphering its behavior and predicting its reactivity.
Concept of Neutrality in Nitrogen Atoms
In its neutral state, nitrogen atoms have a zero oxidation number. This means that the number of electrons in their outermost energy level, known as the valence electrons, is equal to the number of protons in their nucleus. Nitrogen has five valence electrons, and since it has an equal number of protons (also five), its oxidation number in elemental form is zero.
Ionic Bond Formation and its Impact on Oxidation Numbers
When nitrogen atoms participate in ionic bond formation, they either gain or lose electrons, resulting in a nonzero oxidation number. For example, when nitrogen forms an ionic bond with sodium (a metal), nitrogen gains one electron, becoming negatively charged and acquiring an oxidation number of -1. Conversely, when nitrogen bonds with oxygen (a nonmetal), nitrogen loses one electron, becoming positively charged with an oxidation number of +1.
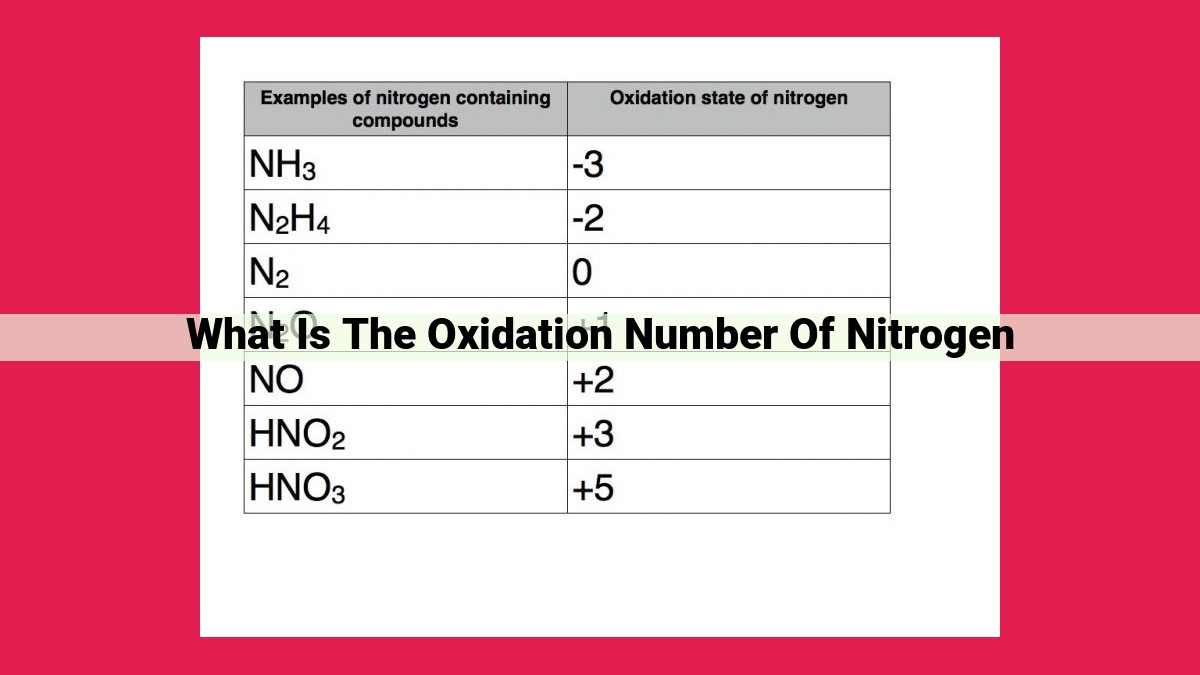
Common Oxidation Numbers of Nitrogen
Nitrogen exhibits a wide range of oxidation numbers, including +5, +4, +3, +2, -3, and -1. These oxidation numbers are commonly encountered in various nitrogen-containing compounds:
- +5: Nitrogen pentoxide (NO5-)
- +4: Nitrogen dioxide (NO2)
- +3: Nitrous acid (HNO2)
- +2: Nitric oxide (NO)
- -3: Ammonia (NH3)
- -1: Sodium nitride (Na3N)
Applications of Oxidation Numbers: Predicting Reactions and Understanding Structure
Oxidation numbers, those deceptively simple numerical values assigned to atoms in molecules, play a pivotal role in predicting the intricacies of chemical reactions and unraveling the enigmatic world of electronic structure.
In the realm of redox reactions, where electrons dance between atoms like ethereal sprites, oxidation numbers serve as guiding stars. By tracking the change in oxidation numbers, chemists can decipher the oxidation and reduction half-reactions that drive the overall reaction. This knowledge empowers us to predict the products of reactions, ensuring the safe and controlled synthesis of countless substances that enrich our lives.
Beyond redox reactions, oxidation numbers shed light on the electronic tapestry of molecules. They reveal the relative electronegativity of atoms, their tendency to attract electrons. This understanding is crucial for comprehending the formation of ionic compounds and the intricacies of crystal structure. By mapping the distribution of charge within molecules, oxidation numbers guide our explorations into the molecular world.
In short, oxidation numbers are indispensable tools for understanding the behavior of chemicals. They provide a lens through which we can glimpse the hidden dynamics of reactions and unravel the mysteries of electronic structure.
Related Concepts: Unveiling the Interplay of Oxidation Numbers
Oxidation State, Electronegativity, and Electron Configuration
- Oxidation state refers to the hypothetical charge that an atom would have if electrons were transferred completely to or from other atoms in a compound.
- Electronegativity measures an atom’s tendency to attract electrons towards itself.
- Electron configuration describes the distribution of electrons in the atom’s orbitals.
These concepts are intertwined: elements with a high electronegativity tend to have a positive oxidation state, indicating a loss of electrons. Conversely, elements with low electronegativity typically have negative oxidation states, signifying a gain of electrons.
Ionic Compounds, Crystal Structure, and Solubility
- Ionic compounds are formed by the transfer of electrons between atoms, resulting in positively charged ions (cations) and negatively charged ions (anions).
- Crystal structure refers to the arrangement of atoms or ions within a solid.
- Solubility measures the ability of a substance to dissolve in a solvent.
Oxidation numbers play a crucial role in understanding the formation and properties of ionic compounds. For example, the oxidation number of nitrogen in sodium nitrate (NaNO3) is +5, indicating that nitrogen has lost five electrons and formed a cation. This knowledge helps us predict the compound’s crystal structure and solubility.