Understanding Nickel’s Valence Electrons: Key To Its Chemical Properties And Behavior
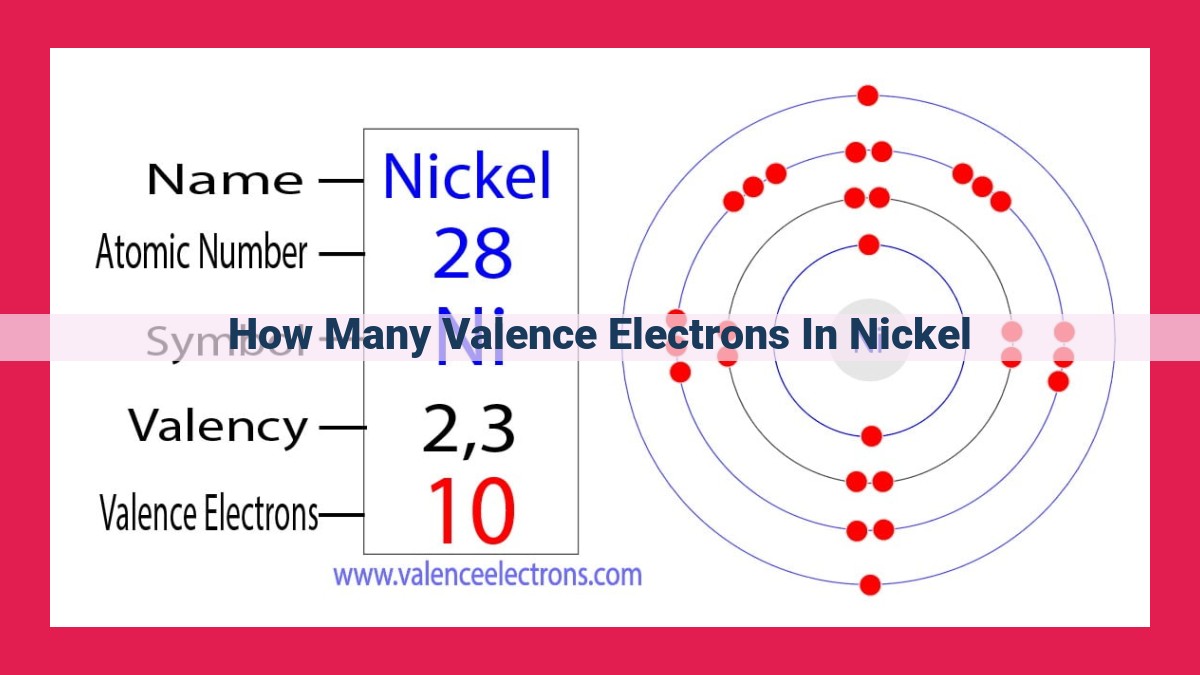
Understanding the number of valence electrons in nickel is crucial for comprehending its chemical properties and behavior. Nickel is a transition metal with an atomic number of 28, placing it in the d-block of the periodic table. Its electron configuration is [Ar] 3d8 4s2, indicating eight valence electrons. These valence electrons occupy the 3d and 4s orbitals, contributing to nickel’s unique characteristics, including incomplete d orbitals, variable oxidation states, and magnetic properties influenced by unpaired electrons.
The Enigmatic Nickel: Unraveling the Secrets of Valence Electrons
In the realm of chemistry, electrons play a pivotal role in shaping the symphony of elements. Valence electrons, those occupying the outermost energy level, are the sorcerers that orchestrate the properties of matter. Understanding the dance of valence electrons is essential to unlocking the secrets of nickel, a metal with an intriguing personality.
Valence Electrons and Nickel’s Symphony
Nickel, an element residing in the transition metal family, proudly displays 10 valence electrons. These electrons, like mischievous sprites, dictate nickel’s unique traits, enabling it to engage in a diverse array of chemical reactions. The number of valence electrons plays a crucial role in determining nickel’s chemical reactivity, magnetic properties, and even its lustrous silvery-white appearance.
Delving into the Electronic Configuration
Nickel’s electronic configuration, the blueprint of its atomic structure, reveals the enigmatic dance of its electrons. With 28 electrons whirling around its nucleus, nickel’s electronic configuration can be unraveled as:
1s²2s²2p⁶3s²3p⁶3d⁸4s²
This intricate arrangement of electrons allows nickel to exhibit its exceptional characteristics. The eight valence electrons in the 3d and 4s orbitals bestow nickel with its magnetic properties, making it a valuable component in magnets and electrical devices.
The Enigmatic Transition Metals
Nickel belongs to the enigmatic group known as transition metals, characterized by their incomplete d orbitals and a propensity to exhibit multiple oxidation states. The presence of these partially filled d orbitals grants transition metals their distinctive versatility, enabling them to participate in a vast array of chemical reactions.
Unveiling the Secrets of d-Block Elements
Nickel’s placement in the d-block of the periodic table further illuminates its intriguing properties. The presence of d electrons significantly influences nickel’s magnetic properties, reactivity, and the formation of complex compounds. These characteristics make nickel an indispensable component in numerous industrial applications, including the production of stainless steel and alloys.
3d Orbitals: The Invisible Architects
The 3d orbitals of nickel, shaped like cloverleaves, play a pivotal role in determining the element’s magnetic properties. The number of unpaired electrons in these orbitals directly influences nickel’s magnetic behavior, allowing it to align itself with magnetic fields. This property makes nickel an essential material in the realm of magnetism, finding applications in everything from refrigerator magnets to MRI machines.
Unpaired Electrons: The Harbingers of Reactivity
The presence of unpaired electrons in nickel’s 3d orbitals bestows upon it an unmatched reactivity. Unpaired electrons act as catalysts, facilitating chemical reactions and allowing nickel to participate in a wide range of chemical processes. This reactivity makes nickel an integral component in industrial applications, such as catalysis, electroplating, and the production of batteries.
Oxidation States: The Versatile Persona of Nickel
Nickel exhibits a remarkable ability to adopt multiple oxidation states, including +2, +3, and +4. These oxidation states arise from the varying number of electrons that nickel can lose or gain. The versatility of nickel’s oxidation states enables it to form a diverse array of compounds, each with its unique properties.
Nickel, with its captivating symphony of valence electrons, embodies the essence of the transition metals. Its enigmatic properties, influenced by its electronic configuration, d-block placement, and unpaired electrons, make nickel an indispensable element in modern society. From its lustrous silvery appearance to its magnetic properties and unmatched reactivity, nickel continues to captivate the curiosity of scientists and engineers alike, inspiring countless discoveries and technological advancements.
The Enigmatic Nature of Nickel: Unraveling the Secrets of Valence Electrons
In the intricate tapestry of the periodic table, nickel stands out as a captivating enigma. Its exceptional properties are intricately linked to the dance of its valence electrons, offering a fascinating glimpse into the world of chemistry.
Unveiling the Electronic Configuration of Nickel
The electronic configuration of an element describes the distribution of its electrons within its atomic orbitals. Nickel, nestled in Group 10 of the periodic table, boasts an atomic number of 28. This means that each nickel atom harbors 28 electrons circling its nucleus.
The unique arrangement of these electrons profoundly influences nickel’s chemical behavior. The outermost electrons, known as valence electrons, play a pivotal role in determining its properties. Nickel possesses eight valence electrons, making it a decidedly transition metal.
Transition Metals: A Versatile Ensemble
Transition metals are defined by their incomplete d orbitals and ability to exhibit variable oxidation states. Nickel, being a transition metal, shares these traits. Its d orbitals are partially filled, rendering it highly reactive and capable of forming bonds with a variety of elements.
The Allure of d-Block Elements
Nickel belongs to the coveted d-block of the periodic table. These elements possess d orbitals, which are crucial for their magnetic and catalytic properties. The presence of d electrons grants nickel its exceptional versatility, allowing it to form a diverse array of compounds.
The electronic configuration of nickel, with its eight valence electrons, profoundly influences its chemical behavior, establishing it as a transition metal and a member of the d-block. These properties endow nickel with its remarkable versatility, making it an indispensable element in a myriad of scientific and industrial applications. Exploring the fascinating world of nickel’s valence electrons unveils the intricate dance of chemistry, revealing the hidden secrets that govern the properties of this remarkable element.
**Transition Metals: A Key to Understanding Nickel’s Properties**
In the realm of chemistry, transition metals stand out as a fascinating and versatile group of elements. Their distinguishing characteristic lies in their incomplete d orbitals, which are filled with electrons but not entirely so. This unique arrangement endows them with remarkable properties, such as variable oxidation states.
Nickel’s Place in the Transition Metal Family
Nickel proudly belongs to the d-block elements and occupies a central position in the periodic table. Its electron configuration, with 28 electrons arranged in specific energy levels, places it among the transition metals. This strategic location grants nickel access to the 3d orbitals, which play a pivotal role in shaping its behavior.
Nickel’s position as a transition metal has a profound impact on its properties. The incomplete d orbitals allow nickel to exist in multiple oxidation states. This versatility makes it a valuable component in alloys and catalytic processes. For instance, nickel’s oxidation state of +2 is commonly found in nickel-plated objects, while its oxidation state of +3 is crucial in the production of stainless steel.
The Significance of d-Block Elements in Shaping the Properties of Nickel
In the realm of elements, transition metals stand out as a captivating group, adorned with d-block elements that hold the key to understanding their unique properties. Nickel, an enigmatic element, belongs to this noble group, and its d electrons play a pivotal role in shaping its very essence.
d-Block Elements: The Building Blocks of Transition Metals
The d-block elements reside in the periodic table’s middle section, holding their place between the s-block and p-block elements. These elements are characterized by their incomplete d orbitals, which are capable of accommodating up to ten electrons. This incomplete electronic configuration gives rise to the variable oxidation states that are a hallmark of transition metals, including nickel.
Influence of d Electrons on Nickel’s Properties
The presence of d electrons profoundly influences the properties of nickel. These electrons occupy 3d orbitals, which possess a unique shape that allows for the formation of complexes with various ligands. This complex-forming ability endows nickel with its catalytic properties, making it an essential component in many industrial and chemical processes.
Additionally, the number of unpaired d electrons determines nickel’s magnetic properties. When paired, d electrons cancel out their magnetic fields, resulting in a non-magnetic material. However, if there are unpaired d electrons, they generate a magnetic field, making nickel ferromagnetic (attracted to magnets). This property is crucial for applications such as electromagnets and permanent magnets.
3d Orbitals: The Essence of Nickel’s Magnetic Charm
The 3d orbitals, a set of five energy levels, exist within the d-block of the periodic table, where nickel resides. These orbitals have a unique shape, resembling a four-leaf clover, and can accommodate up to ten electrons.
In the case of nickel, its electronic configuration is 3d8 4s2. This means that nickel has eight electrons in its 3d orbitals, leaving two in the 4s orbital. These unpaired electrons in the 3d orbitals play a crucial role in determining nickel’s magnetic properties.
The Hund’s rule states that electrons will occupy separate orbitals before pairing up. As a result, the eight electrons in nickel’s 3d orbitals occupy four separate orbitals with one electron each, leaving two unpaired electrons. These unpaired electrons interact with each other, creating a magnetic field.
The magnetic field generated by the unpaired electrons in nickel’s 3d orbitals is what gives nickel its ferromagnetic properties. This means that nickel can be magnetized and attracted to other magnets. The strength of the magnetic field depends on the number of unpaired electrons present, which explains why nickel, with its two unpaired electrons, has a stronger magnetic field than other elements with fewer unpaired electrons.
In summary, the 3d orbitals of nickel play a vital role in determining its magnetic properties. The presence of eight unpaired electrons in these orbitals results in a strong magnetic field, making nickel a valuable material for applications involving magnetism.
Unpaired Electrons: The Key to Understanding Nickel’s Properties
Imagine a bustling city, filled with countless individuals, each with their own unique role to play. In the world of chemistry, the atoms of an element are like these city dwellers, with valence electrons being the ones that actively engage with the world around them. Understanding the number of valence electrons is crucial for deciphering the properties of elements, and for nickel, this knowledge unlocks a deeper appreciation of its remarkable characteristics.
The Concept of Unpaired Electrons
Among its valence electrons, some may exist in a solitary state, known as unpaired electrons. These lone electrons are like unattached individuals, eager to interact with others. The number of unpaired electrons significantly influences the reactivity and magnetic properties of an element.
Nickel and Unpaired Electrons
Nickel, in its elemental form, possesses two unpaired electrons. These electrons reside in its partially filled 3d orbitals, giving nickel its unique magnetic properties. The unpaired electrons allow nickel to align itself with magnetic fields, making it a ferromagnetic material. This property is what gives nickel its magnetic allure, enabling it to be used in applications such as magnets and magnetic alloys.
Reactivity and Unpaired Electrons
The presence of unpaired electrons also has a profound impact on nickel’s reactivity. Unpaired electrons are more readily available for forming bonds with other atoms, making nickel a more reactive element. This reactivity is essential for nickel’s involvement in various chemical reactions, allowing it to form compounds with a wide range of other elements.
Just as unpaired individuals can contribute to the dynamics of a city, unpaired electrons play a pivotal role in shaping the behavior of nickel. Their presence determines nickel’s magnetic properties, influences its reactivity, and underscores its importance in numerous chemical processes. Understanding the concept of unpaired electrons is a key to unlocking the complexities of this versatile element and appreciating its significance in our technological world.
Oxidation States of Nickel
In the realm of chemistry, oxidation states play a pivotal role in unraveling the behavior and reactivity of elements. Nickel, a fascinating transition metal, showcases a rich tapestry of oxidation states, offering a tantalizing glimpse into its versatile nature.
Defining Oxidation States
Oxidation state refers to the hypothetical charge an atom would acquire if all its bonds were purely ionic. It provides a convenient tool for understanding the distribution of electrons in a compound, helping us decipher the chemical dance between elements.
Nickel’s Oxidation States
Nickel exhibits a remarkable range of oxidation states, primarily due to its transition metal status. These states arise from the interplay between its electronic configuration and its tendency to form varying numbers of bonds. The most common oxidation states of nickel are:
-
0 (Zero): Found in elemental nickel, denoting a neutral state where nickel atoms share electrons equally.
-
+2 (Two): The most stable oxidation state for nickel, arising from the loss of two electrons from its outer shell.
-
+3 (Three): A less common state, formed when nickel donates three electrons.
-
+4 (Four): An even less common state, attributed to the loss of four electrons by nickel.
Understanding the Spectrum of Oxidation States
The electronic configuration of nickel, with its incomplete d orbital, provides the key to its diverse oxidation states. The presence of these unpaired d-electrons allows nickel to accommodate varying numbers of electrons, resulting in the spectrum of oxidation states observed.
In the +2 oxidation state, nickel loses two d-electrons, leaving it with a stable, half-filled d orbital. This arrangement lends stability to this oxidation state, making it the most prevalent for nickel.
Impact of Oxidation States on Chemistry
The oxidation state of nickel profoundly influences its chemical reactivity. Different oxidation states exhibit varying tendencies to form specific types of bonds, dictating their participation in diverse chemical reactions. This versatility allows nickel to play a crucial role in a wide range of industrial and catalytic processes.
By delving into the oxidation states of nickel, we uncover the intricacies of its chemistry, paving the way for deeper exploration of this remarkable element.