Understanding Molecular Composition: Building Blocks Of Matter
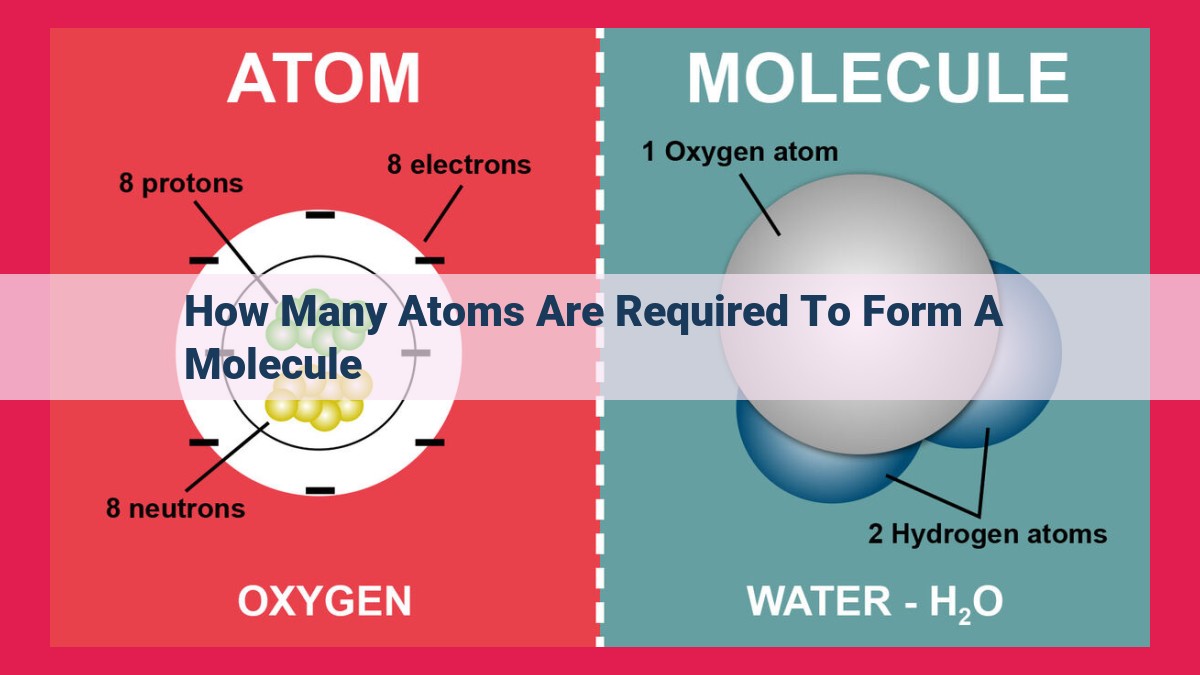
Molecules, the building blocks of matter, are formed by the combination of atoms. The number of atoms required depends on the type of molecule. Diatomic elements consist of two identical atoms (e.g., H2, O2), while polyatomic elements have multiple identical atoms (e.g., S8, P4). The molecular formula, using subscripts and superscripts, represents the composition and number of atoms in a molecule. Subscripts indicate the number of atoms of each element, while superscripts represent polyatomic ions or the charge of molecules. Understanding the composition of molecules is crucial in fields such as chemistry, biology, and materials science.
Understanding Molecules: The Building Blocks of Matter
In the vast tapestry of the universe, everything around us is composed of fundamental units known as molecules. These tiny entities are the building blocks of matter, shaping the world we experience. From the air we breathe to the food we eat, every substance is an intricate dance of molecules.
Molecules are formed when atoms, the smallest particles of an element, combine in specific arrangements. These arrangements are dictated by the unique chemical properties of each element. When atoms come together, they share or exchange their electrons, giving rise to the diverse molecules we encounter.
Diatomic elements are the simplest type of molecule, composed of two identical atoms. Hydrogen gas (H2) and oxygen gas (O2) are classic examples of diatomic elements. These molecules are highly stable due to the strong attraction between their atoms.
Polyatomic elements, on the other hand, are molecules composed of multiple identical atoms. Sulfur (S8) and phosphorus (P4) are examples of polyatomic elements. These molecules can form complex structures and exhibit unique properties, making them essential for various chemical reactions.
Define the three types of molecules: diatomic elements, polyatomic elements, and compounds.
Understanding Molecules: The Building Blocks of Matter
Atoms: The Core of Molecules
Imagine a tiny universe within an atom. At its center lies the nucleus, a densely packed core housing protons (positively charged) and neutrons (neutral). Surrounding the nucleus are electrons, negatively charged particles that zip around in specific paths called orbitals. These electrons determine an atom’s chemical properties.
Diatomic Elements: Molecules of Two
When two identical atoms join, they form a diatomic element molecule. Think of these as playful twins bonded together. Common examples include hydrogen (H2) and oxygen (O2). These diatomic elements are the building blocks of many larger molecules.
Polyatomic Elements: Molecules of Multiple
Some elements don’t settle for one partner. They form polyatomic elements molecules, where multiple identical atoms band together. Sulfur (S8) and phosphorus (P4) are such examples. These molecules exhibit unique properties and often play crucial roles in biological processes.
Molecular Formula: A Chemical Blueprint
Just as architects use blueprints to design buildings, chemists use molecular formulas to depict molecules. These formulas provide a snapshot of a molecule’s composition. For example, the formula for water is H2O, indicating it contains two hydrogen atoms and one oxygen atom.
Subscripts: Counting Atoms in a Molecule
Subscripts in a molecular formula tell us how many atoms of a specific element are present. CO2, for instance, has one carbon atom and two oxygen atoms. Subscripts help us differentiate molecules with similar compositions but different proportions.
Superscripts: Groups and Ions
When molecules get more complex, superscripts come into play. They represent polyatomic ions or indicate the number of ions in a compound. H2SO4 (sulfuric acid) is a prime example, with its superscript 4 denoting the number of sulfate ions (SO4) it carries.
Stoichiometry and the Balance of Reactions
Chemistry is like a dance of atoms and molecules, where balance is key. Stoichiometry helps us understand the precise ratios of reactants (the starting materials) to products (the resulting substances) in chemical reactions. Balanced equations ensure that reactions proceed smoothly, just like a perfectly choreographed dance.
Understanding the world of molecules is like diving into the intricate tapestry of matter. From the simplicity of diatomic elements to the complexities of polyatomic ions, molecules form the foundation of life, medicine, and countless other scientific fields. By deciphering their composition and interactions, we unlock the secrets of our universe, one molecule at a time.
Understanding the Core of Matter: Atoms
In the vast tapestry of the universe, the building blocks of all things are atoms, the fundamental units of matter. Each atom, an intricate universe in itself, is composed of a dense nucleus and a surrounding cloud of electrons. The nucleus, the atom’s beating heart, houses the protons, positively charged particles, and neutrons, their neutral counterparts. These nuclear residents are bound together by the enigmatic force of the strong nuclear interaction, an unyielding bond that governs the very core of existence.
Orbiting around the nucleus, electrons dance in a symphony of motion. Like celestial bodies, they occupy specific energy levels, each a distinct orbit with its own unique character. The innermost energy level, closest to the nucleus, can host a maximum of two electrons, while the outer energy levels have a greater capacity. As we move outwards, each energy level accommodates more electrons, following a geometric progression.
The number of protons within the nucleus determines an atom’s atomic number, a unique identifier that distinguishes one element from another. Elements, the fundamental constituents of the universe, each have their own distinct atomic number, defining their chemical properties and behavior.
The arrangement of electrons within the energy levels influences an atom’s reactivity, determining how it interacts with other atoms. This electronic configuration dictates whether an atom willingly forms bonds or prefers to remain solitary. Understanding the structure of atoms, therefore, is paramount to unraveling the nature of matter and the countless interactions that shape our world.
Understanding Molecules: Unlocking the Secrets of Matter’s Building Blocks
Embark on a captivating molecular journey, where we unravel the enigmatic composition that defines the very essence of matter. In this blog post, we delve into the enchanting world of molecules, their formation, and the remarkable role they play in shaping our universe.
Molecules, the fundamental units of matter, are like the intricate building blocks of our world. Each molecule is a minuscule masterpiece composed of atoms, the basic constituents of matter. Atoms, with their nucleus of protons and neutrons and a surrounding cloud of electrons, act as the essential Lego bricks of molecules. These minuscule particles fuse together in various combinations to create the vast array of molecules we encounter in our daily lives.
Think of a molecule as a microscopic orchestra, with each atom contributing a unique sound to the overall symphony. In diatomic elements, such as the life-sustaining oxygen we breathe (O2) or the explosive hydrogen that fuels rockets (H2), two identical atoms join hands like inseparable twins. Polyatomic elements, like the pungent sulfur (S8) we smell in rotten eggs, involve multiple identical atoms forming complex molecular structures.
Just as words are made up of letters, the language of molecules relies on molecular formulas to convey their composition. These formulas, like chemical blueprints, paint a picture of the molecular architecture. Subscripts, like tiny numbers nestled next to an element symbol, reveal the number of atoms present. Just as the number of bricks determines the size of a house, the number of atoms dictates the molecular framework. For instance, in water (H2O), the subscript 2 indicates two hydrogen atoms uniting with a single oxygen atom.
Superscripts, on the other hand, take on a more dynamic role. They represent groups of atoms, such as polyatomic ions, or indicate the overall charge of a molecule or ion. In sulfuric acid (H2SO4), for example, the superscript 4 signifies the presence of a sulfate ion with a charge of -2, giving the molecule its acidic properties.
By understanding these molecular formulas, we unlock the secrets of chemical reactions, the intricate dance where molecules interact and transform. Stoichiometry, the science of balanced equations, ensures that reactions proceed in harmonious proportions, like a perfectly choreographed ballet. The careful arrangement of reactants and products, represented by their molecular formulas, reveals the quantitative relationships that govern chemical change.
In conclusion, molecules, the enigmatic building blocks of matter, play a pivotal role in sculpting our world. From the air we breathe to the food we consume, molecules weave the fabric of our existence. By delving into their composition and unraveling their molecular formulas, we unlock the secrets of chemistry, gaining profound insights into the very building blocks of our universe.
Understanding Molecules: The Building Blocks of Matter
In the realm of science, the concept of molecules reigns supreme. These minuscule entities are the very essence of matter, the fundamental particles that make up everything in existence. Without them, this vibrant tapestry we call the universe would be an empty void.
What Are Molecules?
Imagine molecules as the building blocks of matter. They are microscopic entities that can’t be further broken down. Each molecule consists of atoms, the even tinier particles that determine a molecule’s properties and behavior.
Types of Molecules
The molecular world is a diverse tapestry woven from three main threads: diatomic elements, polyatomic elements, and compounds. Diatomic elements are the simplest molecules, consisting of just two identical atoms joined together. Examples include hydrogen (H2) and oxygen (O2), the very air we breathe.
Atoms: The Heart of Molecules
Atoms are the core of molecules. Each atom comprises a nucleus, containing protons and neutrons, and electrons, which orbit the nucleus. The number of protons in an atom’s nucleus determines its elemental identity.
Diatomic Elements: Molecules of Two
Diatomic elements are the epitome of simplicity in the molecular world. These molecules are composed of two atoms of the same element, bonded together by a shared electron pair. Hydrogen (H2), the fuel of stars and power plants, is a classic example. Oxygen (O2), the lifeblood of all living creatures, is another.
Properties of Diatomic Elements
Diatomic elements often display unique properties due to their symmetrical structure and strong covalent bonds. Hydrogen, for instance, is highly flammable, while oxygen is essential for cellular respiration. These elements are also typically gaseous at room temperature.
Understanding Molecules and Their Composition: Unlocking the Building Blocks of the Universe
Diatomic Elements: The Molecules of Two
As we delve into the wondrous world of molecules, let’s encounter the simplest of them all: diatomic elements. These are the fundamental units of matter that consist of two identical atoms joined together in a molecular embrace.
Picture hydrogen (H2), the most abundant element in the universe, as two shy atoms holding hands. They’ve decided to form a bond because, like all atoms, they seek stability and a complete outer electron shell. In oxygen (O2), two oxygen atoms intertwine, creating the molecule essential for life on our planet.
Diatomic elements possess unique properties that set them apart. For instance, hydrogen burns with a pale, almost invisible flame, while oxygen is responsible for the vibrant blue color of a Bunsen burner. These special characteristics arise from the specific arrangements of electrons within the diatomic molecules.
By understanding the behavior and properties of diatomic elements, we uncover the secrets of the molecular world. Whether it’s understanding the composition of air we breathe or the chemistry behind combustion, these tiny duos play a pivotal role in the symphony of nature.
Polyatomic Elements: Molecules Comprised of Many
Venture into the realm of polyatomic elements, molecules that embody the essence of unity. Unlike diatomic elements with their cozy twosome, polyatomic elements embrace a multitude of identical atoms, forging a cohesive molecular entity.
Picture sulfur (S8), a vibrant yellow element that adorns our chemistry sets. Its polyatomic nature manifests as a ring of eight sulfur atoms, intertwined to form a stable, symmetrical structure. Similarly, phosphorus (P4) captivates with its tetrahedral geometry, its four atoms arranged in a perfect pyramid.
Polyatomic elements possess unique properties that distinguish them from their diatomic counterparts. Their enlarged molecular size and complex architecture often result in lower reactivity and higher melting and boiling points. These traits make polyatomic elements essential components of various industrial processes and scientific applications.
Unlocking the secrets of polyatomic elements is crucial to comprehending the diversity and complexity of the molecular world. From the intricate structures of proteins to the reactive properties of acids and bases, polyatomic elements play a pivotal role in shaping our understanding of chemistry and its vast applications.
Polyatomic Elements: A Molecular Symphony of Many
Polyatomic elements, a fascinating class of molecules, consist of multiple identical atoms harmoniously bonded together. Unlike diatomic elements, which feature a duet of atoms, polyatomic elements embrace a more intricate molecular arrangement. Their unique properties arise from the complex interplay between multiple atoms within their structures.
Consider the sulfur molecule, S8, composed of a ring of eight sulfur atoms. This molecular configuration endows sulfur with a distinctive yellow color, low melting point, and high reactivity. Similarly, the phosphorus molecule, P4, exhibits a tetrahedral shape, resulting in its low melting point and ability to form various allotropes, including white, red, and black phosphorus.
Polyatomic elements play crucial roles in various chemical processes. For instance, oxygen, a diatomic molecule, is essential for respiration and combustion, while ozone, a polyatomic molecule composed of three oxygen atoms, serves as a protective layer in the Earth’s atmosphere. These intricate molecular architectures demonstrate the remarkable diversity and complexity of polyatomic elements.
Explain the concept of a molecular formula and its role in representing the composition of molecules.
Molecular Formula: The Chemical Blueprint
In the realm of chemistry, molecules are the fundamental players, the microscopic building blocks of everything that exists. To decipher the makeup of these molecular marvels, scientists have devised a special code known as a molecular formula.
Just as a blueprint reveals the design of a house, a molecular formula serves as a detailed roadmap to a molecule’s composition. It spells out the identity and quantity of each atom, which is the tiniest indivisible unit of an element.
The elements themselves are like elemental ingredients, each with its own unique symbol. Hydrogen (H), oxygen (O), and nitrogen (N) are some of the most common. By stringing together these elemental symbols with subscripts and superscripts, we can create molecular formulas that tell us exactly how many of each atom are present.
For instance, the molecular formula H20 reveals that a water molecule consists of two hydrogen atoms (H subscripts) and one oxygen atom (O). This formula not only identifies water but also provides a quantitative understanding of its composition.
Molecular formulas are like the Rosetta Stones of chemistry, unlocking the secrets of molecular structure. They guide us in understanding the properties and reactivity of molecules, enabling us to delve into the intricate world of chemical reactions. By deciphering molecular formulas, we gain invaluable insights into the very fabric of matter.
Understanding Molecules and Their Composition: Unraveling the Building Blocks of Matter
Imagine the world as a vast tapestry woven together by tiny threads called molecules. These fundamental units of matter form the very essence of everything we see, touch, and interact with. Molecules come in various flavors, but scientists have classified them into three main categories: diatomic elements, polyatomic elements, and compounds.
Atoms: The Core of Molecules
At the heart of every molecule lies an atom, the smallest unit of matter that still retains its chemical identity. Atoms consist of a dense nucleus harboring protons and neutrons, surrounded by a swarm of electrons. These electrons arrange themselves in specific energy levels, determining an atom’s unique properties.
Diatomic Elements: Molecules of Two
Diatomic elements are the simplest molecules, consisting of just two identical atoms. Think of hydrogen (H2) and oxygen (O2), both composed of pairs of the same element. These diatomic molecules exhibit distinct characteristics that reflect the properties of their individual atoms.
Polyatomic Elements: Molecules of Multiple
Polyatomic elements are molecules made up of multiple identical atoms. Here, we encounter fascinating substances like sulfur (S8) and phosphorus (P4). These polyatomic molecules possess unique properties that arise from the way their multiple atoms interact.
Molecular Formula: A Chemical Blueprint
To represent the composition of molecules, scientists use molecular formulas. These formulas are like chemical blueprints that reveal the exact arrangement of atoms in a molecule. Subscripts and superscripts play crucial roles in interpreting these formulas.
Subscripts: Counting Atoms in a Molecule
Subscripts are numbers placed beside elements in a molecular formula. They tell us the number of atoms of that particular element in the molecule. For instance, H2O indicates that each water molecule contains two hydrogen atoms (H) and one oxygen atom (O).
Superscripts: Groups and Ions
Superscripts, on the other hand, are numbers placed above elements in a molecular formula. They often represent polyatomic ions or the number of ions in a compound. For example, the molecular formula H2SO4 tells us that each molecule contains two hydrogen ions (H+), one sulfate ion (SO42-), and a total charge of -2.
Stoichiometry and the Balance of Reactions
Understanding molecules is essential in unraveling the mysteries of chemical reactions. Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical equation. Balanced equations ensure that the number of atoms of each element is the same on both sides of the reaction, reflecting the law of conservation of mass.
Understanding Molecules: The Building Blocks of Our World
In the realm of chemistry, molecules reign supreme as the fundamental units of matter. They form the very essence of everything around us, from the air we breathe to the water we drink. Understanding molecules and their composition is crucial for unlocking the secrets of our world.
Atoms: The Core of Molecules
Every molecule is made up of tiny particles called atoms. An atom is like a solar system, with a central nucleus surrounded by orbiting electrons. The nucleus contains protons and neutrons, while the electrons whizz around it in specific energy levels. Atoms are the fundamental building blocks of molecules, just as bricks are to a building.
Subscripts: Counting Atoms in Molecules
When atoms combine to form molecules, they do so in specific ratios. A subscript in a molecular formula indicates the number of atoms of a particular element in that molecule. For example, in the formula H2O, the subscript “2” tells us that there are two hydrogen atoms for every one oxygen atom. This ratio is essential for understanding the molecule’s properties and its role in chemical reactions.
Examples of Subscripts
- Sodium chloride (NaCl): This molecule contains one sodium atom for every one chlorine atom.
- Water (H2O): This molecule contains two hydrogen atoms for every one oxygen atom.
- Carbon dioxide (CO2): This molecule contains one carbon atom for every two oxygen atoms.
Subscripts are indispensable tools for deciphering the composition of molecules. They allow us to determine the exact number of atoms present, which is vital for understanding the molecule’s structure, properties, and reactivity. By mastering the use of subscripts, we gain a deeper insight into the building blocks of our world.
The Building Blocks of Our World: Understanding Molecules and Their Composition
In the realm of science, where the tiniest of particles dance upon the stage of discovery, molecules emerge as the fundamental building blocks of all matter. They are the very essence of our existence, shaping the world around us in ways both visible and invisible.
What is a Molecule?
Imagine a molecule as an intricate puzzle, its pieces interlocking seamlessly. Each piece represents an atom, the indivisible core of an element. When two or more atoms join forces, they form a molecule, creating a structure imbued with unique properties.
Types of Molecules: A Trio of Vielfalt
The molecular world is a diverse tapestry, adorned with three primary types of molecules:
-
Diatomic Elements: These molecules are like cosmic twins, composed of two identical atoms. Think of the vital oxygen we breathe (O2) or the combustible hydrogen that powers our rockets (H2).
-
Polyatomic Elements: Imagine nature’s building blocks in abundance. Polyatomic elements consist of multiple identical atoms, like the enigmatic phosphorus (P4) or the ethereal sulfur (S8).
-
Compounds: These molecules are the embodiment of harmony, combining atoms of different elements. They form the intricate structures that govern life and create the familiar substances we encounter daily, such as water (H2O) or carbon dioxide (CO2).
The Molecular Formula: A Chemical Blueprint
Each molecule carries its own unique identity, expressed through its molecular formula. This formula serves as a blueprint, revealing the exact number and arrangement of atoms within the molecule. For instance, the molecular formula of glucose, the sugar that fuels our bodies, is C6H12O6.
Subscripts: Counting the Atomic Ensemble
Subscripts in a molecular formula play a crucial role in specifying the number of atoms of each element. In H2O, the subscript 2 indicates that there are two hydrogen atoms for every one oxygen atom.
Superscripts: Ions and More
Superscripts come into play when dealing with polyatomic ions or the number of ions in compounds. For example, in sulfuric acid (H2SO4), the superscript 4 denotes that there are four sulfate ions (SO42-) per two hydrogen ions (H+).
Stoichiometry: The Balancing Act of Reactions
Stoichiometry, the chemistry of proportions, unravels the numerical relationships between reactants and products in chemical reactions. It ensures that reactions proceed in a balanced manner, ensuring that neither atoms nor elements are lost or gained. This intricate dance of stoichiometry underpins the countless chemical processes that sustain life and drive technological advancements.
Molecules: The Invisible Fabric of Our World
From the proteins that orchestrate life’s processes to the plastics that shape our material world, molecules are the unseen architects that govern our reality. Understanding their composition and properties is not merely an academic pursuit but a key to unlocking the secrets of nature and harnessing its potential for the betterment of humankind.
Superscripts: Navigating Polyatomic Ions and Compound Charges
In the realm of molecules, superscripts serve as powerful tools, unlocking secrets hidden within the intricate tapestry of elements and their interactions. As we’ve explored the concept of molecular formulas, we’ve encountered these enigmatic symbols, standing tall like beacons of information.
Superscripts, often seen gracing the upper right corners of elements, hold a profound significance in the language of chemistry. They beckon us to a deeper understanding of molecules, guiding us through the maze of polyatomic ions and the intricate balance of ionic compounds.
- Polyatomic Ions: Guardians of Charge
Polyatomic ions, the enigmatic molecules that grace chemical formulas, come equipped with their own distinct charges. Superscripts step into the spotlight, revealing these charges with unwavering precision. For instance, the superscript ‘2-‘ adorning the sulfate ion (SO₄²⁻) proclaims its possession of two negative charges.
- Ions in Compounds: Unveiling the Dance of Charges
Superscripts also play a pivotal role in deciphering the intricate dance of ions in compounds. By carefully observing the superscript, we can determine the number of ions present, providing invaluable insights into the compound’s overall charge. Take, for example, the compound calcium chloride (CaCl₂). The superscript ‘2’ indicates that two chloride ions (Cl⁻) have joined forces with a single calcium ion (Ca²⁺), creating a neutral compound.
Understanding superscripts unlocks a treasure trove of knowledge about molecules and their interactions. They reveal the hidden charges lurking within polyatomic ions and illuminate the subtle dance of ions in compounds. As we continue our journey into the molecular world, superscripts will serve as our unwavering companions, guiding us through the intricacies of chemistry.
Describe how superscripts represent the overall charge of ions or molecules (e.g., H2SO4).
Superscripts: Unraveling the Secrets of Electrically Charged Molecules
In the world of molecules, not all are neutral players. Some possess an electrical charge, imbuing them with unique properties and behavior. To understand these charged molecules, we turn to the humble superscript, an essential character in the chemical language.
Superscripts, often seen perched above a chemical formula, are not mere bystanders. They carry a profound significance: they reveal the overall charge of ions or molecules. Ions are atoms or molecules that have lost or gained electrons, resulting in a net positive or negative charge.
For example, consider the enigmatic H2SO4. This seemingly innocuous formula conceals a powerful secret: its superscript of “4” holds the key to its ionic nature. The “4” signifies that the molecule carries a negative charge of 4. This charge arises from the presence of four hydrogen ions (H+) and one sulfate ion (SO42-). The hydrogen ions surrender their electrons, leaving the sulfate ion with a surplus of negative charge, which is then balanced by the positively charged hydrogen ions.
Superscripts play a crucial role in deciphering the behavior of charged molecules. They reveal their ability to attract or repel other charged particles, influencing their interactions and chemical reactions. Understanding the significance of superscripts empowers us to delve deeper into the intricate world of electrochemistry, where charged molecules dance and react, shaping the molecular tapestry of our universe.
Understanding Molecules: The Building Blocks of Our Universe
Molecules, the fundamental units of matter, are the invisible architects behind everything we can touch, taste, smell, and experience. They determine the properties of substances and drive the reactions that shape our world. Join us on a molecular adventure as we delve into their composition, structure, and role in chemical reactions.
Atoms: The Essence of Matter
At the heart of molecules lie atoms, the tiny building blocks of the universe. Each atom consists of a nucleus, housing protons and neutrons, surrounded by a cloud of electrons. The arrangement of these subatomic particles defines an atom’s unique identity and its potential for forming molecules.
Molecules: Bonds and Combinations
When atoms dance together, they create molecules through chemical bonds. These bonds, like molecular superglue, hold atoms in place, forming sturdy molecular structures. Diatomic elements, such as hydrogen and oxygen, are molecules made of two identical atoms. Polyatomic elements, like sulfur and phosphorus, consist of multiple identical atoms joined together.
Molecular Formula: A Chemical Blueprint
Every molecule has a unique molecular formula, a blueprint that reveals its atomic composition. Subscripts denote the number of atoms of a particular element, while superscripts indicate the number of ions or polyatomic groups within the molecule. For instance, the formula H₂SO₄ tells us that sulfuric acid contains two hydrogen atoms, one sulfur atom, and four oxygen atoms.
Stoichiometry: Balancing the Molecular Equation
Chemical reactions, the dance of molecules, follow the principle of stoichiometry. Stoichiometry, like a molecular accountant, ensures that the number of atoms and molecules on the reactants’ side of a chemical equation matches the number on the products’ side. Balanced chemical equations help us predict the amounts of reactants and products involved in a reaction, unlocking the secrets of chemical transformations.
Understanding molecules and their composition is the key to unlocking the mysteries of science, medicine, and materials science. From drug discovery to materials design, molecules play a vital role in shaping our world. By delving into their structure and interactions, we gain a deeper appreciation for the molecular tapestry that weaves the fabric of our universe.
Describe the role of balanced equations in representing the quantitative relationships between reactants and products.
Understanding Molecules and Their Composition: A Journey into the Heart of Matter
At the core of everything we see and touch lies the invisible world of molecules, the fundamental units of matter. Molecules are tiny entities that bond together like microscopic puzzles, creating all the substances we know and interact with. Understanding the nature and composition of molecules is crucial for unraveling the secrets of the physical world.
Atoms: The Core of Molecules
Every molecule is constructed from atoms, the smallest indivisible units of chemical elements. Atoms have a central nucleus, made up of positively charged protons and neutral neutrons, surrounded by negatively charged electrons. The arrangement of electrons in shells around the nucleus determines the atom’s properties and its ability to combine with others.
Diatomic Elements: Molecules of Two
Diatomic elements are molecules composed of two identical atoms joined by a strong chemical bond. These atoms share electrons to achieve a stable configuration, resulting in molecules with distinct properties. Hydrogen (H2) and oxygen (O2) are classic examples of diatomic elements.
Polyatomic Elements: Molecules of Multiple
Polyatomic elements are molecules composed of multiple identical atoms bonded together. Instead of sharing electrons, the atoms in polyatomic elements form strong covalent bonds, creating molecules with unique physical and chemical characteristics. Sulfur (S8) and phosphorus (P4) are examples of polyatomic elements.
Molecular Formula: A Chemical Blueprint
A molecular formula is a shorthand notation that represents the composition of a molecule. It indicates the types and numbers of atoms present in the molecule. Subscripts denote the number of atoms of a particular element, while superscripts represent the number of ions or polyatomic groups. For example, H2O represents a water molecule, with two hydrogen atoms and one oxygen atom.
Stoichiometry and the Balance of Reactions
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Balanced equations are essential mathematical expressions that represent these relationships. Balancing equations ensures that the number of atoms of each element remains the same on both sides of the equation, reflecting the conservation of mass. For example, the balanced equation for the combustion of methane (CH4) is:
CH4 + 2O2 → CO2 + 2H2O
This equation shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
Understanding molecules and their composition is a fundamental step toward unraveling the fabric of our universe. From the simplest diatomic molecules to complex organic compounds, molecules play a central role in every aspect of our lives, influencing the properties of materials, the reactions that occur in our bodies, and the very air we breathe. By delving into the world of molecules, we unlock the secrets of the physical world and gain a deeper appreciation for the intricate beauty of nature.
Delving into the Molecular Realm: Unraveling the Secrets of Matter
In the vast tapestry of our universe, matter exists in countless forms, each with its own unique nature. At the very foundation of matter lie molecules, the fundamental building blocks that determine the properties and behavior of everything that surrounds us.
Atoms: The Core Building Blocks
Molecules, though seemingly simple entities, are actually intricate structures composed of even smaller units: atoms. Atoms are the fundamental particles of matter, each possessing a nucleus at its heart. This nucleus is a dense concentration of protons and neutrons, while electrons orbit around the nucleus in distinct shells. The arrangement of electrons determines the atom’s chemical behavior and its ability to combine with other atoms, forming molecules.
Diatomic Elements: Molecules of Unity
The simplest type of molecule is a diatomic element, composed of two identical atoms bound together. Hydrogen (H2) and oxygen (O2) are prime examples. These diatomic molecules often exhibit distinct properties that differ from their individual atoms. For instance, hydrogen is a colorless, odorless gas, while hydrogen gas is highly flammable.
Polyatomic Elements: Molecules of Multiplicity
Polyatomic elements are akin to diatomic elements but consist of multiple identical atoms joined together. Examples include sulfur (S8) and phosphorus (P4). These molecules showcase unique characteristics that stem from the interactions between the constituent atoms.
Molecular Formula: The Chemical Blueprint
Scientists use molecular formulas to depict the precise composition of molecules. These formulas utilize subscripts to indicate the number of atoms of each element present. For example, the formula for water (H2O) reveals that each molecule contains two hydrogen atoms and one oxygen atom.
Superscripts: Unveiling Charge and Structure
Superscripts play a crucial role in representing polyatomic ions or the number of ions in a compound. For instance, the formula for sulfuric acid (H2SO4) employs a superscript to indicate the presence of one sulfate ion (SO42-) and two hydrogen ions (H+).
Stoichiometry: Balancing the Equation
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. By analyzing balanced equations, scientists can determine the exact amounts of reactants required to produce a given amount of product. This knowledge is essential for myriad applications, from pharmaceutical formulations to industrial processes.
Our journey into the realm of molecules has unveiled the intricate nature of matter and the remarkable interplay between atoms and their arrangements. Understanding molecules and their composition is not merely an academic pursuit but a gateway to unraveling the mysteries of our world and unlocking countless possibilities in science, technology, and medicine.
Understanding Molecules and Their Composition: A Cornerstone of Scientific Inquiry
Molecules are fundamental building blocks of the universe. They form the foundation of matter, from the air we breathe to the water we drink. Understanding their composition and properties is crucial for a comprehensive grasp of science and its myriad applications.
Each molecule possesses a unique blueprint, its molecular formula, which reveals the exact arrangement and number of atoms it contains. These formulas unlock insights into the molecule’s behavior, reactivity, and significance.
Atoms, the subatomic components of molecules, play a critical role in determining their properties. By unraveling the structure and dynamics of atoms, scientists gain a deeper understanding of the nature of matter and the chemical reactions that shape our world.
Diatomic elements, composed of two identical atoms, and polyatomic elements, made up of multiple identical atoms, showcase the diverse molecular configurations that exist. Their unique properties, such as stability or reactivity, contribute to the chemical diversity of our planet.
Beyond their theoretical importance, understanding molecules and their composition has practical implications in various scientific fields and applications:
-
Chemistry: Molecules serve as the basis for chemical reactions, determining the formation of new substances and the release or absorption of energy.
-
Biology: The structure and function of proteins, carbohydrates, and lipids – the building blocks of life – hinge upon the interactions of molecules.
-
Medicine: Drugs, developed to interact with specific molecules, rely on precise knowledge of molecular composition for targeted and effective treatments.
-
Materials science: Understanding molecular composition enables the design of new materials with tailored properties, from lightweight alloys to advanced electronic devices.
Unveiling the secrets of molecules and their composition empowers us with a deeper appreciation for the complexity and elegance of the natural world. From unraveling the mysteries of the human body to shaping technological advancements, the study of molecules continues to mold our understanding and unlock new possibilities for scientific exploration.