Understanding Ionization: Key To The Fundamental Properties Of Acids
- Ionization: The Essence of Acids
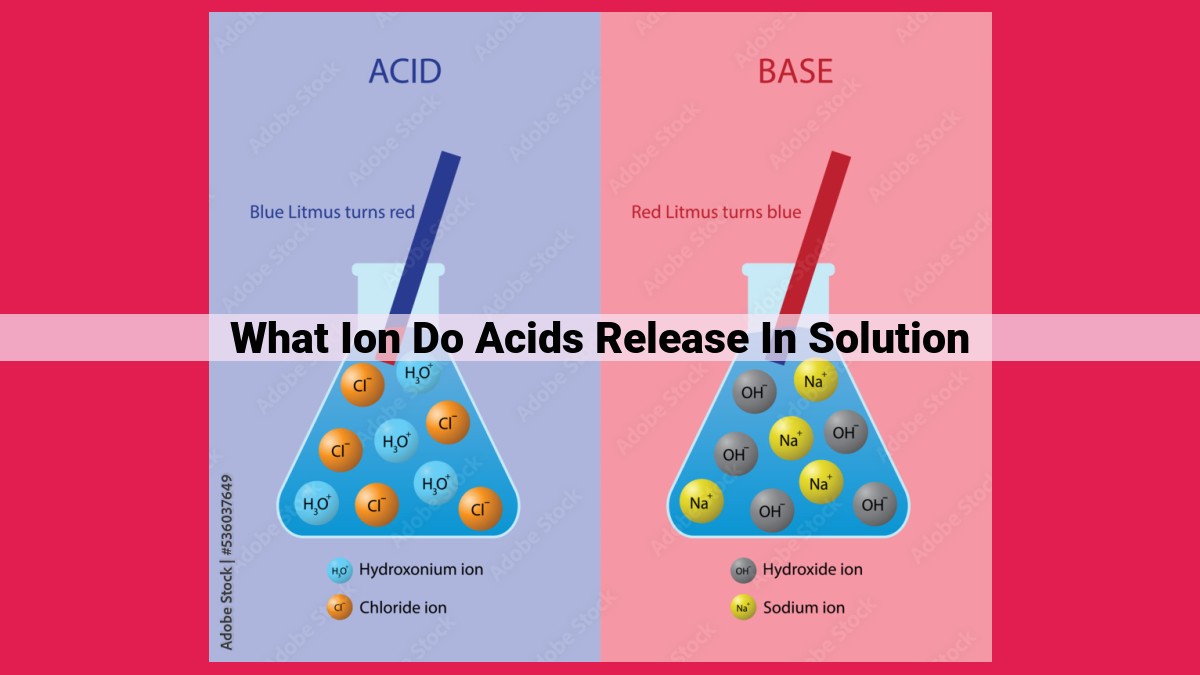
Acids, when dissolved in solutions, undergo ionization, releasing hydrogen ions (H+). These H+ ions determine the acidity of solutions, with higher concentrations resulting in greater acidity (lower pH). pH, a measure of acidity-alkalinity, ranges from 0 to 14, with acids having a pH below 7. Concentration, typically expressed as molarity, is inversely related to pH, indicating the amount of H+ ions present. Furthermore, concentration influences density, with higher concentrations generally leading to higher densities.
Ionization: The Core of Acids
Acids play a ubiquitous role in our world, from the zesty tang of lemons to the industrial might of batteries. Ionization, the fundamental process that defines acids, holds the key to understanding their unique properties.
In the realm of acids, ionization is the process where molecules break apart into ions, electrically charged atoms or molecules. The defining characteristic of acids lies in their ability to release hydrogen ions (H+) when dissolved in water. These liberated hydrogen ions are the driving force behind the characteristic sour taste and corrosive nature of acids.
The extent of ionization, and thus the acidity of a solution, hinges on the concentration of hydrogen ions. The higher the concentration of hydrogen ions, the more acidic the solution. This relationship is inversely reflected in the concept of pH, a measure of the acidity of solutions that ranges from 0 to 14. A lower pH indicates a higher concentration of hydrogen ions and, thereby, greater acidity. Conversely, a higher pH denotes a lower concentration of hydrogen ions and, consequently, a less acidic solution.
Hydrogen Ion: The Acidity Determinant
In the fascinating world of acids, hydrogen ions (H+) reign supreme as the key players that define their acidic nature. These tiny particles, armed with their positively charged personalities, embark on a mission to determine the acidity of solutions, leaving an undeniable mark on our daily lives.
A Souring Experience: The Role of Hydrogen Ions in Taste
Ever wondered why some foods taste sour? It’s all thanks to the presence of hydrogen ions. These acidic warriors sneak into our taste buds and unleash a symphony of sensations, stimulating the sour receptors and leaving a puckering impression on our palates. The higher the concentration of hydrogen ions, the more intense the sourness we perceive.
Corrosive Nature: Hydrogen Ions Unleash Their Might
Acids aren’t just limited to tantalizing our taste buds. They also possess a more formidable side, as their hydrogen ions wield the power of corrosion. When acids encounter certain materials, like metals, their hydrogen ions react with them, forming new compounds and potentially damaging the material’s structure. This corrosive behavior makes acids valuable in industrial settings but also necessitates caution in their handling.
The Gatekeepers of Acidity: Hydrogen Ions and pH
The acidity of a solution is expressed through a numerical scale called pH, which ranges from 0 to 14. The pH value is inversely proportional to the hydrogen ion concentration: the higher the hydrogen ion concentration, the lower the pH. This means that highly acidic solutions have low pH values, while less acidic or alkaline solutions have higher pH values. Understanding pH is crucial in various fields, including environmental monitoring, medical diagnostics, and the food industry.
By unraveling the significance of hydrogen ions, we gain a deeper appreciation for the diverse and impactful role acids play in our world. From shaping our culinary experiences to fueling industrial processes, hydrogen ions are the unsung heroes behind the acidic realm.
Concentration: The Key to pH
In the realm of acids, the dance of hydrogen ions (H+) reigns supreme. These tiny powerhouses determine the acidity of a solution, leaving an indelible mark on our taste buds and the fabric of our world.
pH, a number that quantifies the acidity or alkalinity of a solution, serves as a crucial yardstick in this chemical ballet. It scales from 0 to 14, with lower values indicating greater acidity.
At the heart of pH lies the concentration of hydrogen ions. As the concentration of these ions increases, the pH decreases, signaling a more acidic environment. Conversely, a lower concentration of hydrogen ions translates to a higher pH, indicating lower acidity or even an alkaline state.
This inverse relationship between hydrogen ion concentration and pH is of paramount importance in understanding acid behavior. It allows us to quantify the acidity of solutions, whether they be the tangy bite of lemon juice or the corrosive power of industrial acids.
By understanding the role of concentration in pH, we gain a window into the world of acids, unlocking their properties and enabling us to harness their power for a multitude of practical applications.
pH: The Acidity-Alkalinity Indicator
In the realm of chemistry, the concept of pH plays a pivotal role in understanding the behavior and properties of various substances. pH is an indispensable tool for scientists, environmentalists, and medical professionals alike, offering a crucial lens through which to decipher the acidity or alkalinity of a solution.
What is pH?
pH stands for “potential of hydrogen” and represents the concentration of hydrogen ions (H+) present in a solution. This dimensionless measure exists on a scale ranging from 0 to 14, with a neutral solution having a pH of 7. Solutions with a pH below 7 are deemed acidic, while those with a pH above 7 are considered alkaline (or basic).
The Significance of pH
pH serves as a fundamental indicator of a solution’s acidity or alkalinity. Highly acidic solutions (pH less than 7) possess a greater concentration of hydrogen ions, giving them a sour taste and corrosive nature. Conversely, highly alkaline solutions (pH greater than 7) have a lower concentration of hydrogen ions, resulting in a bitter taste and slippery feel.
Applications of pH
The determination of pH finds widespread application in numerous fields.
- Environmental Monitoring: Measuring pH is crucial for assessing the health of aquatic ecosystems and soil quality. It helps identify pollutants that can alter the acidity of water bodies and damage plant life.
- Medical Diagnostics: pH plays a vital role in diagnosing certain medical conditions, such as acid reflux, kidney disease, and cystic fibrosis. Blood pH, for instance, provides insights into a patient’s respiratory and metabolic status.
- Food Science: pH is essential in the preservation and safety of food products. Controlling pH inhibits the growth of spoilage microorganisms and ensures optimal texture and flavor.
- Industrial Processes: pH is monitored in industrial settings to optimize chemical reactions, prevent corrosion, and control wastewater treatment.
pH, as a measure of hydrogen ion concentration, provides a powerful tool for understanding the acidity or alkalinity of solutions. Its applications span a diverse range of fields, enabling scientists, environmentalists, and medical professionals to make informed decisions and gain valuable insights into various processes and substances.
Related Concepts: Molarity and Density
To fully grasp the nature of acids and their impact on our world, we must delve into two additional concepts: molarity and density.
Molarity: A Measure of Concentration
Molarity measures the concentration of a solution, specifically the number of moles of solute (in this case, acid) per liter of solvent. It’s a key indicator of how strong an acid solution is.
Density: A Reflection of Concentration
Density refers to the mass of a substance per unit volume. Interestingly, there is a relationship between concentration and density. Typically, higher concentrations lead to higher densities. This means that denser acid solutions contain more dissolved acid molecules.
By understanding these related concepts, we gain a more comprehensive understanding of the properties and behavior of acids. Molarity helps us determine the exact strength of an acid solution, while density provides insights into its physical characteristics.