Understanding Ion Formation: Charged Atoms, Valence Electrons, And Electron Transfer
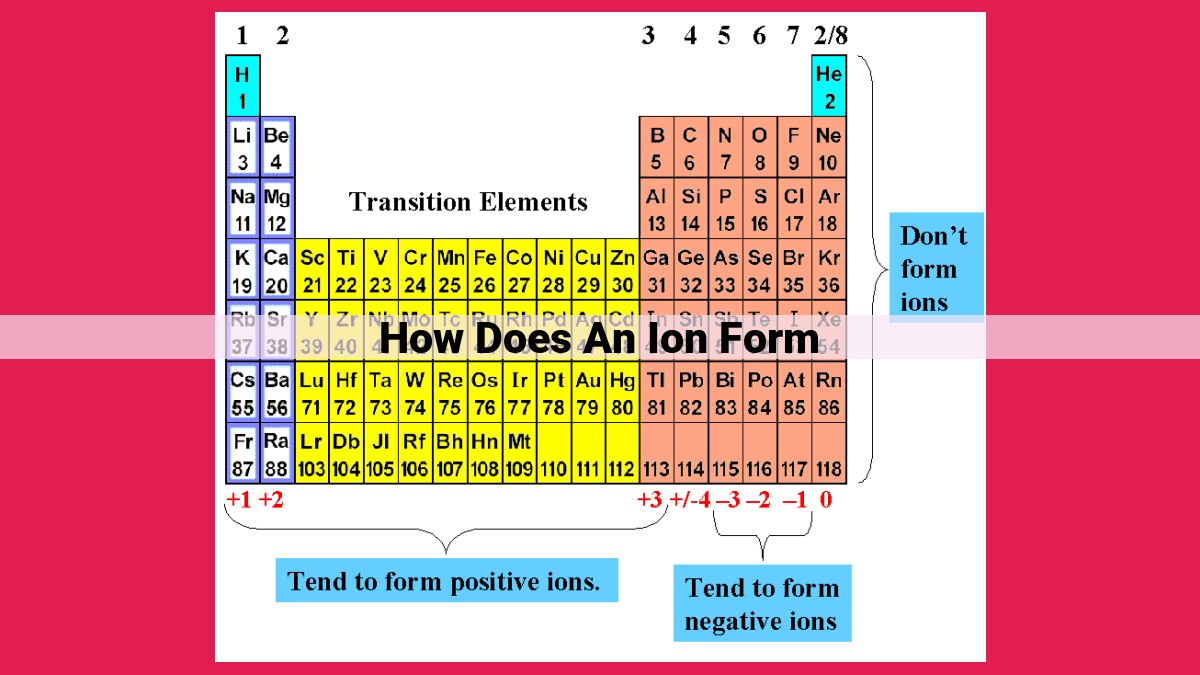
Ionization occurs when atoms or molecules lose or gain electrons, resulting in charged species called ions. The process involves changes in electron configuration, particularly the outermost valence electrons. When an atom loses electrons, it becomes a positively charged cation, while gaining electrons leads to the formation of a negatively charged anion. Oxidation and reduction are key processes involved in ion formation, where electrons are transferred between atoms or molecules. Understanding the concepts of charged atoms, electron configuration, and valence electrons is crucial for comprehending ion formation and its impact on atomic and molecular properties.
Understanding Ionization: The Cornerstone of Chemistry
In the realm of chemistry, ionization stands as a fundamental process that shapes the behavior and properties of atoms and molecules. It is the act of gaining or losing electrons, creating a world of electrically charged entities known as ions. To unravel the complexities of this fascinating phenomenon, let’s embark on a journey through its key concepts.
Charged Ions: A Balance Unequal
When an atom or molecule loses or gains electrons, it becomes electrically charged. This imbalance creates the two main types of ions: cations and anions. Cations are positively charged, while anions carry a negative charge.
Electron Configuration: The Atomic Blueprint
The arrangement of electrons around an atom’s nucleus is known as its electron configuration. This blueprint determines an atom’s chemical reactivity and ionization potential. Ionization disrupts electron configuration, altering an atom’s stability and reactivity.
Valence Electrons: The Gateway to Ionization
Valence electrons are the outermost electrons in an atom, responsible for chemical bonding. These electrons play a pivotal role in ionization. Atoms tend to gain or lose electrons to achieve a stable electron configuration, often involving valence electrons.
Oxidation and Reduction: The Ion-Forming Duo
Oxidation and reduction are two inseparable processes that can lead to ionization. Oxidation involves the loss of electrons, while reduction entails gaining them. These electron transfers can result in the formation of ions, with oxidized species forming cations and reduced species forming anions.
Charged Atoms or Molecules: A Matter of Balance
In the realm of chemistry, atoms and molecules strive to maintain an equilibrium in their electrical charge. When this balance is disrupted, charged atoms or molecules emerge, paving the way for the formation of ions.
The Unequal Dance of Electrons and Protons
Atoms, the fundamental building blocks of matter, are composed of a dense nucleus surrounded by orbiting electrons. The nucleus harbors protons, positively charged particles, while electrons carry a negative charge. In their natural state, atoms exist in a state of electrical neutrality, with the number of electrons precisely matching the number of protons.
However, when an atom loses or gains electrons, it acquires a charge. Atoms that lose electrons become positively charged, while those that gain electrons attain a negative charge. These charged atoms are known as ions.
Cations and Anions: The Positives and Negatives
Among ions, two distinct types emerge: cations and anions. Cations are positively charged ions, formed when atoms lose electrons. In this process, the number of protons exceeds the number of electrons, resulting in a net positive charge. Conversely, anions are negatively charged ions, created when atoms gain electrons. Here, the number of electrons surpasses the number of protons, leading to a net negative charge.
The formation of ions is driven by a quest for stability. When an atom loses or gains electrons, it adjusts its electron configuration to achieve a more stable arrangement. This electron rearrangement often leads to the formation of ionic bonds between oppositely charged ions, further solidifying the existence of charged atoms and molecules.
Electron Configuration: Mapping the Atomic Landscape
The blueprint of an atom, its electron configuration, unveils the arrangement of electrons in distinct energy levels orbiting the nucleus. These electrons, like celestial bodies, occupy specific orbits based on their energy. When an atom sheds or gains electrons through ionization, this delicate dance is subtly altered.
Imagine an atom as a celestial sphere, its nucleus a glowing sun. Electrons, like planets, orbit this nucleus in well-defined energy levels. Each level represents a different distance from the nucleus, and the farther an electron is from the nucleus, the higher its energy.
Electron configuration is like a celestial map, charting the distribution of electrons in these energy levels. The first energy level, closest to the nucleus, can hold a maximum of two electrons, while the second level can accommodate up to eight. As we move farther away from the nucleus, these energy levels increase in size and capacity.
Ionization, the process of adding or removing electrons from an atom, disrupts this celestial dance. When an atom gains electrons, its electron configuration expands, with new electrons occupying higher energy levels. Conversely, when an atom loses electrons, its electron configuration contracts, leaving empty spaces in the energy levels.
Understanding electron configuration is crucial for comprehending the behavior and reactivity of atoms. It helps us predict the types of ions an atom can form, determine the chemical bonds it can participate in, and ultimately unravel the complexities of the chemical world.
Valence Electrons: The Gateway to Ion Formation
In the realm of chemistry, the dance of electrons orchestrates the creation of ions, the charged particles that shape the molecular landscape. Enter valence electrons, the pivotal players in this captivating ballet.
Residing in the outermost energy level of an atom, valence electrons are the gatekeepers of chemical reactivity. They determine an atom’s propensity to gain or lose electrons, a process known as ionization.
The loss of valence electrons unveils a positively charged cation, while their gain gives rise to a negatively charged anion. This electron exchange brings atoms into a harmonious balance, creating the charged species that drive countless chemical reactions.
Valence electrons are the gatekeepers of an atom’s oxidation state, a measure of the number of electrons lost or gained. The transition from a neutral atom to an ion involves a change in oxidation state, with the loss of electrons leading to a more positive oxidation state and the gain of electrons resulting in a more negative oxidation state.
Comprehension of the role of valence electrons in ion formation paves the path to understanding the intricate tapestry of chemical reactions. It unlocks the secrets of chemical bonds, molecular transformations, and the myriad of phenomena that shape the world around us.
Oxidation and Reduction: The Two Sides of the Coin
- Explain the processes of oxidation (loss of electrons) and reduction (gain of electrons).
- Show how these processes can lead to the formation of ions.
Oxidation and Reduction: The Two Sides of the Ionization Coin
In the world of chemistry, atoms and molecules are constantly interacting, forming new substances and breaking down old ones. Among these interactions are the processes of oxidation and reduction, which involve the transfer of electrons. These processes are like two sides of a coin, with one face being oxidation (the loss of electrons) and the other being reduction (the gain of electrons).
As electrons shift from one atom or molecule to another, they can create charged species known as ions. Oxidation occurs when an atom or molecule loses electrons, resulting in a positively charged ion called a cation. Conversely, reduction occurs when an atom or molecule gains electrons, resulting in a negatively charged ion called an anion.
The transfer of electrons during oxidation and reduction is a key factor in the formation of chemical compounds and the occurrence of chemical reactions. It’s like a chemical dance where atoms and molecules exchange electrons, creating new ionic species that have different properties and reactivities.
Understanding oxidation and reduction is essential for understanding the fundamental principles of chemistry. These processes play a crucial role in countless chemical reactions that occur in our world, from the rusting of iron to the photosynthesis of plants.
Cations: Positively Charged Ions
- Provide a clear definition of a cation as a positively charged ion.
- Explain how cations are formed and discuss their properties.
Cations: Positively Charged Ions
In the captivating world of ions, we encounter cations, the intrepid knights of the atomic realm. These gallant ions possess a positive charge, the result of their brave decision to shed an electron. Cations embody the essence of chivalry, valiantly sacrificing their negative electrons to restore balance and harmony.
The formation of cations is a tale of sacrifice and transformation. Picture a noble atom, its electrons gracefully orbiting the nucleus like celestial dancers. But sometimes, in the pursuit of cosmic equilibrium, an atom must make a choice: surrender one of its prized electrons. This act of oxidation bestows upon the atom a new identity, transforming it into a cation.
Properties of Cations
Like valiant warriors, cations display remarkable characteristics. Their positive charge grants them a distinct attraction to negatively charged ions, the virtuous maidens of the ionic realm. Cations, with their unwavering positive aura, form strong electrostatic bonds with anions, creating stable compounds that enhance the stability of matter.
Cations also exhibit a remarkable ability to dissolve in water, becoming hydrated ions. In this state, they unleash their power to conduct electricity, making them essential components in batteries and other electrochemical devices. Their electrical prowess empowers them to drive essential processes in living organisms, regulating nerve impulses and muscle contractions.
Examples of Cations
- Sodium (Na+): This gallant sodium cation is renowned for its role in maintaining fluid balance and nerve function.
- Potassium (K+): This steadfast cation plays a crucial role in regulating heart rhythm and muscle contraction.
- Calcium (Ca2+): The ever-reliable calcium cation is indispensable for bone health and cellular signaling.
Cations, the positively charged ions, stand as unwavering pillars of the ionic kingdom. Their formation through oxidation bestows upon them unique properties that enable them to form stable bonds, conduct electricity, and contribute to the very fabric of life. As we delve deeper into the fascinating world of ions, the tale of cations continues to inspire and intrigue, showcasing the intricate dance of atoms and the remarkable forces that shape our universe.
Anions: Negatively Charged Ions
In the realm of chemistry, charged particles called ions play a pivotal role in the formation of compounds and the flow of electricity. Among these ions, anions stand out as negatively charged species that contribute significantly to various chemical reactions and biological processes.
Defining Anions
An anion is an ion that carries a negative charge. This negative charge arises when an atom or molecule gains one or more electrons, resulting in an excess of electrons compared to protons. Anions are often denoted by the chemical symbol of the element followed by a superscript indicating the number of negative charges, such as Cl⁻ for a chlorine anion with one negative charge.
Formation of Anions
Anions are formed when an atom or molecule accepts electrons. This can occur through various mechanisms, including:
- Ionic bonding: When a metal atom loses electrons to a non-metal atom, the non-metal atom gains those electrons and becomes an anion. For example, in sodium chloride (NaCl), sodium atoms lose an electron to chlorine atoms, forming Na⁺ cations and Cl⁻ anions.
- Covalent bonding: In some covalent compounds, one atom can donate a pair of electrons to form a bond with another atom. If the electronegativity of the donating atom is higher than that of the receiving atom, the donated electrons are shifted towards the donating atom, creating a partial negative charge.
- Redox reactions: In redox reactions, one species undergoes oxidation (loss of electrons) while another species undergoes reduction (gain of electrons). The species that gains electrons becomes an anion.
Properties of Anions
Anions possess unique properties that distinguish them from cations and neutral atoms:
- Negative charge: Anions carry a net negative charge due to the excess of electrons.
- Size: In general, anions are larger than their corresponding cations because the added electrons occupy more space.
- Reactivity: Anions are generally more reactive than cations due to their excess of electrons, which makes them more likely to participate in chemical reactions.
- Solubility: Anions form soluble compounds with cations, contributing to the formation of ionic solutions.
Significance of Anions
Anions play crucial roles in various aspects of chemistry and biology:
- Acid-base reactions: Anions contribute to the formation of acids and bases. For example, hydroxide ions (OH⁻) are responsible for the basic properties of solutions.
- Buffering: Anions can act as buffers, helping to maintain a stable pH in solutions.
- Biological processes: Anions are essential for many biological processes, such as the functioning of enzymes and ion channels. For example, chloride ions (Cl⁻) are vital for nerve function.
Anions, as negatively charged ions, hold significance in the world of chemistry and biology. Their unique properties and reactivity contribute to the formation of compounds, the flow of electricity, and the functioning of various biological systems. Understanding the nature and behavior of anions enhances our understanding of chemical processes and their impact on the world around us.