Understanding The Inverse Relationship Between Wavelength And Energy: Implications In Physics, Astronomy, And Medicine
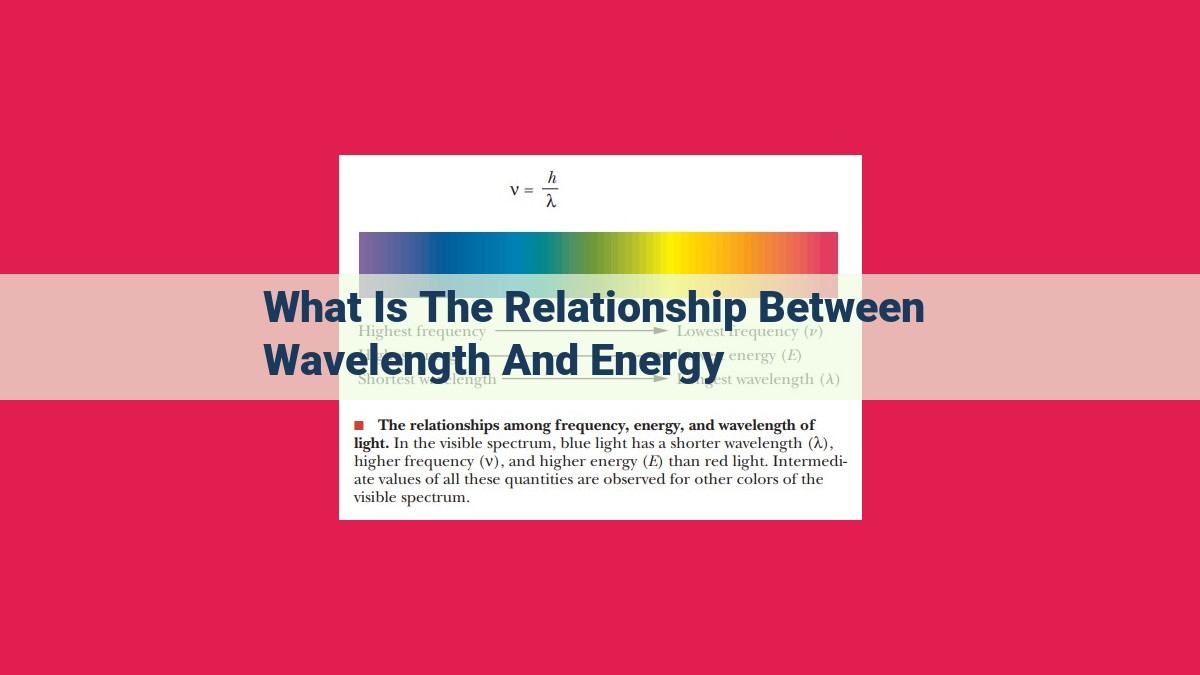
The relationship between wavelength and energy is inverse, where shorter wavelengths correlate with higher energy, and longer wavelengths correspond to lower energy. This concept is evident in the electromagnetic spectrum, with gamma rays having very short wavelengths and immense energy, while radio waves possess long wavelengths and minimal energy. Planck’s constant quantifies this relationship, linking energy to frequency and wavelength. Understanding this inverse relationship is essential in fields like physics, astronomy, and medicine, enabling scientists to interpret phenomena such as blackbody radiation, stellar spectra, and medical imaging.
The Inverse Relationship: A Dance of Wavelength and Energy
In the realm of physics, a fascinating dance unfolds between two fundamental properties: wavelength and energy. These two quantities share an intricate inverse relationship, where the rhythm of their interplay dictates the energy levels and characteristics of various forms of electromagnetic radiation.
Longer Wavelengths, Less Energy: A Gentle Embrace
Imagine a gentle ocean wave, its crest and trough stretching lazily across a wide expanse. This graceful wave represents radiation with a longer wavelength. As the wave’s wavelength stretches, its energy diminishes, just like a whisper fading into the distance.
Shorter Wavelengths, Higher Energy: A Vibrant Symphony
In contrast, think of a high-pitched note, its energetic vibrations creating a rapid succession of peaks and valleys. This vibrant note represents radiation with a shorter wavelength. As the wavelength compresses, its energy intensifies, like the pounding beat of a drum.
Understanding the Inverse Rhythm
This inverse relationship between wavelength and energy is a fundamental law of nature. As one parameter increases, the other inevitably decreases. The longer the wavelength, the lower the energy; the shorter the wavelength, the higher the energy. This dance of opposites forms the foundation of the electromagnetic spectrum.
Electromagnetic Spectrum: A Symphony of Energy
The electromagnetic spectrum is a vast tapestry of radiation, encompassing a wide range of wavelengths and energies. From low-energy radio waves to high-energy gamma rays, each type of electromagnetic radiation occupies a specific region within this spectrum, with its unique wavelength-energy combination.
Cosmic Dancers: Gamma Rays and Radio Waves
At the extreme ends of the spectrum reside the celestial dancers: gamma rays and radio waves. Gamma rays, with their minuscule wavelengths, possess immense energy, capable of penetrating matter with ease. Radio waves, on the other hand, have the longest wavelengths and lowest energy, gently permeating their surroundings.
Light’s Graceful Variations: Infrared and Ultraviolet
In the middle of the spectrum, we encounter infrared and ultraviolet radiation. Infrared radiation, with its longer wavelength, carries less energy than visible light, while ultraviolet radiation, with its shorter wavelength, packs more energetic punch.
Planck’s Constant: The Orchestrator of Energy, Frequency, and Wavelength
Like a cosmic conductor, Planck’s constant harmonizes the relationship between energy, frequency, and wavelength. Its equation (E = hc/λ) establishes a direct connection, where energy (E) is inversely proportional to wavelength (λ). This mathematical elegance governs the dance of wavelength and energy, ensuring their harmonious coexistence.
Significance of the Inverse Relationship
Understanding this inverse relationship is crucial in various scientific fields. In physics, it helps unravel the mysteries of quantum mechanics. In astronomy, it guides the exploration of cosmic phenomena. In medicine, it underpins imaging technologies such as X-rays and MRI scans.
As we delve deeper into the world of physics, let us embrace the intricate dance of wavelength and energy. It is a testament to the profound interconnectedness of the universe, where every physical property plays a role in the grand symphony of nature.
Electromagnetic Spectrum: A Spectrum of Energy
- Introduce the electromagnetic spectrum and its organization.
- Describe the arrangement of electromagnetic waves based on wavelength and energy.
Electromagnetic Spectrum: A Symphony of Energy
Prepare yourself for an enthralling journey through the enigmatic realm of the electromagnetic spectrum, where energy and wavelength dance in an intricate waltz.
Imagine a boundless tapestry woven from an infinite array of electromagnetic waves, each possessing a unique energy and wavelength. This captivating spectrum encompasses the full range of radiation, from the ephemeral whisper of radio waves to the relentless assault of gamma rays.
The dance between energy and wavelength is mesmerizing. As the wavelength elongates, the energy carried by the wave diminishes, like a fading sonata. Conversely, as the wavelength contracts, the energy surges, reaching its crescendo with gamma rays, the most energetic and penetrating form of radiation.
Within this celestial orchestra, we find a multitude of melodies. The sedate rhythm of radio waves lulls us to tranquility, while the energetic strumming of microwaves quickens our pace. Infrared waves bathe us in gentle warmth, their soothing chords resonating with life. The vibrant notes of visible light paint our world with color, from the ethereal glow of violets to the fiery passion of reds. Ultraviolet waves, with their invisible but potent energy, play a vital role in the tapestry of life.
But it is at the extremes of the spectrum where the symphony truly reaches its zenith. Gamma rays, born from the heart of celestial storms, possess an energy so intense that they can shatter atoms, carving paths through matter like celestial lightning bolts. At the opposite end, radio waves, with their unassuming nature, traverse vast cosmic distances, carrying whispers of distant stars and galaxies.
Understanding this intricate dance between energy and wavelength is a key that unlocks the secrets of the universe. It is a language spoken by physicists, astronomers, and physicians alike, enabling them to unravel the mysteries of matter, explore the vastness of space, and heal human bodies.
So, let us embrace this symphony of energy, where wavelengths paint the canvas of our world and energy orchestrates the dance of the cosmos. May this journey through the electromagnetic spectrum inspire a newfound appreciation for the intricate beauty that surrounds us.
Unveiling the Inverse Relationship: Wavelength and Energy
Shorter Wavelengths, Higher Energy: Unveiling the Electromagnetic Spectrum’s Hierarchy
Within the vast expanse of the electromagnetic spectrum, a fascinating inverse relationship unfolds: wavelength and energy engage in a cosmic dance. As wavelengths diminish, energy ascends, painting a vivid canvas of electromagnetic radiation, each with its unique character.
Take gamma rays, for instance. Their wavelengths are minuscule, mere picometers, a testament to their awe-inspiring energy levels. On the opposite end of the spectrum reside radio waves, whose wavelengths stretch to kilometers. Their energy, in contrast, is considerably lower, like the gentle whisper of distant stars.
This inverse relationship dictates the behavior of electromagnetic waves throughout the spectrum. As we traverse from gamma rays to radio waves, we witness a gradual increase in wavelength and a corresponding decrease in energy. It’s a testament to the interconnected nature of the electromagnetic realm, where wavelength and energy are inextricably intertwined.
Longer Wavelengths, Lower Energy: Infrared vs. Ultraviolet
Continuing our exploration of the inverse relationship between wavelength and energy, let’s delve into the fascinating realms of infrared and ultraviolet radiation.
As we venture further down the electromagnetic spectrum, wavelengths lengthen, and energy wanes. This phenomenon becomes evident when we compare infrared and ultraviolet radiation.
Infrared radiation, with its longer wavelengths, possesses lower energy than visible light. It emanates from warm objects, lending it the ability to sense heat. Thermal imaging cameras capitalize on this property, allowing us to “see” heat patterns.
On the other end of the spectrum, ultraviolet radiation boasts shorter wavelengths, translating into higher energy. Naturally found in sunlight, UV radiation is responsible for the warmth we feel on sunny days. However, excessive exposure can be detrimental to our skin and eyes.
This inverse relationship is crucial in understanding various scientific principles and technological applications. In physics, it underpins the wave-particle duality of light. In astronomy, it helps determine the temperatures of distant stars and galaxies. And in medicine, it enables diagnostic techniques like ultrasound and MRI.
By grasping the inverse relationship between wavelength and energy, we unlock a deeper understanding of the electromagnetic spectrum and its profound implications in our world.
Planck’s Constant: Unraveling the Interplay of Energy, Frequency, and Wavelength
In the captivating world of physics, light and other electromagnetic waves dance across a spectrum of wavelengths and energies. Understanding the intricate relationship between these properties is paramount to unraveling the secrets of our universe. Enter Planck’s constant, a fundamental constant that serves as a bridge connecting energy, frequency, and wavelength.
Planck’s constant, denoted by the symbol h, is a tiny but mighty quantity, a mere 6.63 x 10^-34 joule-seconds. Its equation, E = hc/λ, elegantly encapsulates the inverse relationship between energy (E), frequency (c), and wavelength (λ). This equation unveils the profound truth that as wavelength increases, energy decreases, and vice versa.
This inverse relationship manifests itself throughout the vast expanse of the electromagnetic spectrum. Radio waves, with their sprawling wavelengths, possess lower energies, while gamma rays, boasting ultra-short wavelengths, carry immense energy. Infrared radiation, often associated with heat, has longer wavelengths and lower energies compared to ultraviolet radiation’s shorter wavelengths and higher energies.
Planck’s constant plays a pivotal role in diverse scientific fields. In physics, it aids in determining the energy of photons, the fundamental particles of light. Astronomers rely on it to calculate the frequency of light emitted by celestial bodies, providing insights into their composition and dynamics. In medicine, it finds application in imaging techniques like magnetic resonance imaging (MRI), where understanding the relationship between energy and frequency is crucial for generating detailed anatomical images.
Delving into the realm of Planck’s constant is akin to embarking on a cosmic adventure. It unveils the intricate dance between energy, frequency, and wavelength, a dance that orchestrates the tapestry of our physical world. From the smallest subatomic particles to the grandest celestial bodies, Planck’s constant is a constant companion, a beacon guiding our understanding of the universe’s fundamental fabric.
Understanding the Inverse Relationship: Its Importance in Various Fields
In the realm of physics, understanding the inverse relationship between wavelength and energy holds immense significance. This understanding unveils the nature of light and electromagnetic radiation, enabling us to decipher the universe. For instance, in astronomy, observing the wavelengths of light emitted by stars provides valuable insights into their temperature and composition. This knowledge allows scientists to classify stars and study their evolutionary processes.
Beyond physics, this relationship finds applications in medicine as well. Medical imaging techniques such as X-rays and MRI scans rely on the inverse relationship to produce images of internal body structures. X-rays, with their shorter wavelengths and higher energy, can penetrate dense tissues, making them ideal for bone imaging. In contrast, MRI scanners utilize longer wavelength radio waves to create detailed images of soft tissues, as these waves penetrate deeply without causing harm.
Furthermore, the concept of wavelength and energy is crucial in understanding the properties of various electromagnetic waves. From the energetic gamma rays with their extremely short wavelengths to the gentle radio waves with their long wavelengths, the electromagnetic spectrum encompasses a vast range of frequencies and energies. This understanding forms the foundation for technologies like wireless communication, radar systems, and medical treatments using radiation therapy.
By comprehending the inverse relationship between wavelength and energy, we unlock a deeper understanding of the world around us. This knowledge empowers us to explore the depths of the universe, diagnose and treat medical conditions, and harness the power of electromagnetic waves in countless ways. Therefore, grasping this fundamental concept is essential for anyone seeking to unravel the mysteries of light and energy.