Understanding The Inverse Relationship Between Wavelength And Energy In Electromagnetic Waves
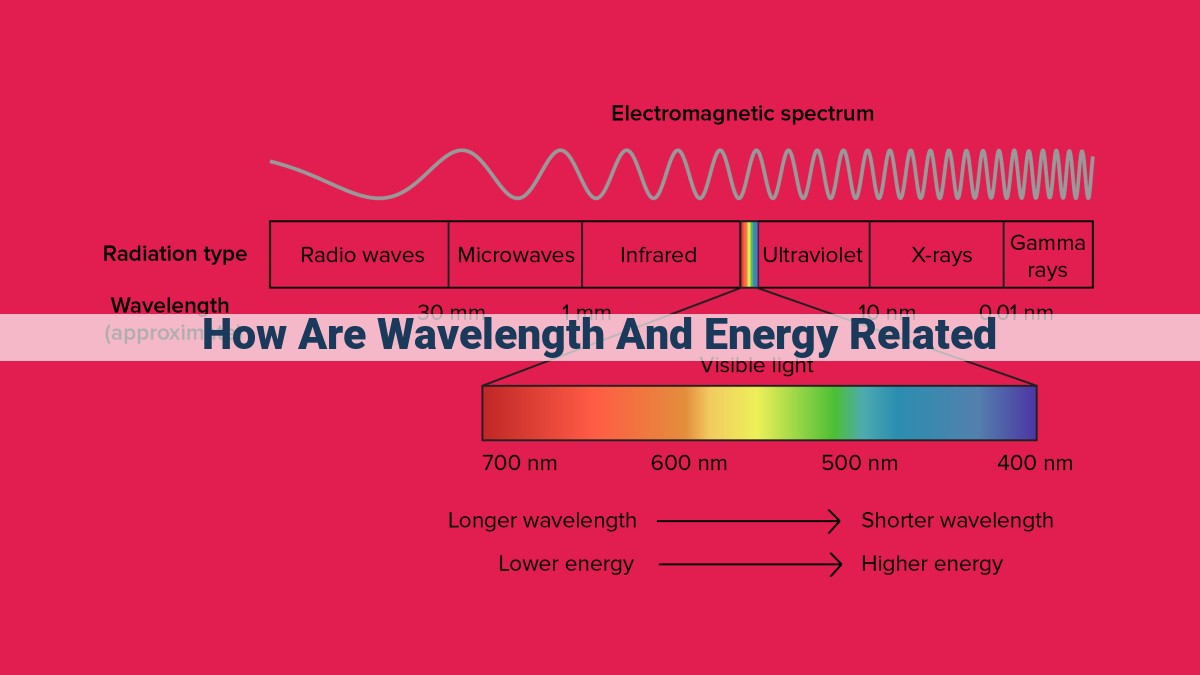
Wavelength and energy are inversely proportional in the world of electromagnetic waves. This means that as the wavelength increases, the energy decreases. The relationship is defined by Planck’s constant: E = hc/λ, where E is energy, h is Planck’s constant, c is the speed of light, and λ is wavelength. This inverse relationship highlights the wave-particle duality of light, as shorter wavelengths correspond to high-energy photons, while longer wavelengths correspond to low-energy photons. Understanding this relationship helps explain phenomena in the electromagnetic spectrum, from gamma rays to radio waves.
- Define wavelength and energy and explain their fundamental nature.
The Dance of Wavelength and Energy: Unraveling the Symphony of Light
In the captivating tapestry of nature’s artistry, the interplay between wavelength and energy forms the very essence of light, the medium that paints our world in vibrant hues and orchestrates the symphony of the cosmos. Wavelength, measured in units of nanometers, represents the distance between successive crests or troughs of a wave, while energy, measured in electron volts (eV), quantifies its potency. Together, they embark on a graceful dance, revealing the profound principles that govern the behavior of light and electromagnetic waves.
At the heart of this dance lies an inverse proportional relationship between wavelength and energy. As the wavelength shortens, the energy of the wave increases. This captivating correlation is elegantly expressed by Planck’s constant, a fundamental constant of nature, which serves as the messenger between these two entities. The mathematical equation that encapsulates this relationship is:
E = hc / λ
where E represents the energy of the wave, h is Planck’s constant, c is the speed of light, and λ is the wavelength.
This inverse proportionality dances in harmony with the wave-particle duality of light, a concept that defies the traditional dichotomy of particles and waves. Light, in its enigmatic nature, exhibits both particle-like and wave-like characteristics. It consists of quanta of energy called photons, each carrying a discrete amount of energy that is inversely proportional to the wavelength of the associated wave. This quantum nature of light forms the cornerstone of our understanding of its behavior and interactions.
The photon energy equation further illuminates the relationship between wavelength and energy. It states that the energy of a photon is directly proportional to its frequency, which, in turn, is inversely proportional to the wavelength. This intricate dance extends beyond the visible spectrum, encompassing a vast electromagnetic spectrum that spans from low-energy radio waves and microwaves to high-energy gamma rays and X-rays. Each region of the spectrum corresponds to specific applications and phenomena, from communication to medical imaging and astrophysical observations.
This interplay between wavelength and energy holds profound implications for our perception of the world. The human eye, a marvel of nature’s engineering, is sensitive to a narrow range of wavelengths within the visible light spectrum. This spectrum, spanning from red to violet, allows us to discern the myriad colors that paint our surroundings.
In conclusion, the relationship between wavelength and energy is a fundamental aspect of light and electromagnetic waves, providing a lens through which we can understand the behavior of these enigmatic entities in our universe. From the duality of light to the organization of the electromagnetic spectrum, this dance of wavelength and energy orchestrates the very fabric of reality, illuminating our perception of the world and fueling our scientific explorations.
Direct Relationship between Wavelength and Energy:
- Present the inverse proportional relationship between wavelength and energy.
- Introduce Planck’s constant and the formula that expresses the relationship.
The Intimate Dance of Wavelength and Energy
In the captivating realm of physics, where light and electromagnetic waves seamlessly intertwine, a fundamental dance unfolds—the relationship between wavelength and energy. Imagine a delicate balance, where each entity mirrors the other, shaping the very fabric of our existence.
An Inverse Embrace
At the heart of this relationship lies an inverse proportionality: as the wavelength of a wave grows, its energy gracefully diminishes. Conversely, when the wavelength contracts, energy ascends, revealing the interconnectedness of these two fundamental properties.
Planck’s Constant: A Gateway to Understanding
Guiding us through this cosmic dance is a constant of nature—Planck’s constant. This enigmatic value, denoted by the enigmatic symbol h, weaves together wavelength and energy in a harmonious equation:
E = hc/λ
Here, E represents the energy of the wave, λ its wavelength, and c the unwavering speed of light. This equation becomes a roadmap, illuminating the path between the two ever-shifting entities.
A Symphony of Waves
From the gentle hum of radio waves to the penetrating gaze of gamma rays, the electromagnetic spectrum paints a symphony of waves, each characterized by its unique wavelength and energy. As we ascend the spectrum, wavelengths diminish, while energy surges. Gamma rays, with their minuscule wavelengths, possess the highest energy, capable of penetrating matter and unlocking the secrets of radioactive cores.
Wave-Particle Duality: The Enigma of Light
Light, a ubiquitous force that permeates our universe, has always captivated scientists. In the realm of physics, light’s true nature has been a subject of intense debate and experimentation. One of the most fundamental aspects of light is its dualistic nature, a paradox where it behaves as both a wave and a particle, blurring the boundaries between these seemingly disparate states.
The wave-like characteristics of light are evident in phenomena such as diffraction and interference, where it exhibits properties similar to other waves. On the other hand, light also displays particle-like behavior, particularly in interactions with matter. These particle-like properties are quantized, meaning that the energy of light is bundled into discrete packets called photons.
The concept of photon energy is crucial in understanding the dual nature of light. Each photon possesses a specific amount of energy proportional to the frequency of the light. The higher the frequency, the more energetic the photon. This relationship is mathematically expressed by the equation E = hf, where E represents photon energy, h is Planck’s constant, and f is frequency.
The quantization of photon energy has profound implications. It means that light energy is not continuous but rather exists in discrete units. This realization has revolutionized our understanding of light and paved the way for advancements in quantum physics.
The wave-particle duality of light has been a source of both fascination and confusion. It challenges our classical notions of reality and raises questions about the fundamental nature of matter and energy. However, it also provides a glimpse into the enigmatic realm of quantum physics, where seemingly contradictory properties can coexist harmoniously.
Photon Energy Equation: The Interplay of Frequency and Wavelength
In the realm of light and electromagnetic waves, wavelength and energy are intertwined in a dance of inverse proportionality. This relationship, elegantly captured in the photon energy equation, unveils the duality of light and its enigmatic quantum nature.
The photon energy equation, also known as Planck’s equation, paints a vibrant picture:
E = hf
where:
- E represents the energy of a photon, the indivisible quantum of light.
- h is Planck’s constant, a fundamental constant of nature.
- f denotes the frequency of the light wave.
This equation whispers a secret: the higher the frequency of light, the higher its energy. Conversely, lower frequencies dance with lower energies. This dance extends an invitation into the captivating world of quantum physics, where energy exists in discrete packets, or quanta.
Equally mesmerizing is the inverse relationship between frequency and wavelength. As frequency ascends, wavelength descends, and vice versa. This delicate balance reveals the intricate tapestry of the electromagnetic spectrum, a symphony of frequencies that weave through the universe.
From Gamma to Radio: The Electromagnetic Spectrum
The electromagnetic spectrum, a harmonious blend of wavelengths and energies, paints a breathtaking canvas of cosmic wonders. At one end resides the enigmatic gamma rays, the cosmic couriers of extraordinary energy and minuscule wavelength. As we traverse the spectrum, we encounter X-rays, known for their piercing power and medical applications.
Descending in energy, we discover the realm of ultraviolet light, a vital force for life on Earth. Visible light, the colors that dance before our eyes, occupies a narrow band of the spectrum, each hue singing a unique wavelength symphony. Beyond visible light, the ethereal elegance of infrared waves unfolds, extending our vision into the thermal realm.
Continuing our journey, we encounter microwaves, the culinary wizards that warm our meals and enhance communication. Finally, at the far end of the spectrum, radio waves blanket the world in their embrace, connecting us across continents and guiding ships through vast oceans.
Each region of the electromagnetic spectrum holds a unique story, a testament to the intricate relationship between wavelength and energy. From the cosmic ballet of gamma rays to the everyday wonders of radio waves, this dance continues to mesmerize scientists and inspire countless innovations.
**High-Energy Waves: The Short and Powerful**
In the realm of electromagnetic waves, energy and wavelength dance in an intricate tango. As energy surges, wavelength shrinks, resulting in a symphony of high-energy, short-wavelength waves. These celestial performers are the masters of penetrating matter and unleashing their potent forces.
Gamma Rays: The undisputed champions of the electromagnetic spectrum, gamma rays reign supreme with their miniscule wavelengths and colossal energy levels. They are the remnants of nuclear reactions, cosmic explosions, and the enigmatic depths of black holes. Their penetrating power is unmatched, enabling them to pierce through thick walls and even the human body, leaving a trail of ionizing radiation.
X-Rays: Next in line are the enigmatic X-rays, the secret weapon of medical diagnostics. Their shorter wavelengths than visible light allow them to peer through skin and bones, revealing hidden fractures and ailments within our bodies. But beware, their high energy can also damage delicate tissues if not handled with utmost care.
These high-energy waves find diverse applications in various fields. Gamma rays are indispensable in cancer treatment, targeting malignant cells with their destructive force. X-rays revolutionized medicine, providing a non-invasive window into our internal landscapes. Their industrial prowess extends to material analysis, revealing hidden defects and ensuring structural integrity.
Their power and presence extend beyond the confines of Earth. In the cosmos, they are the heralds of enigmatic celestial events, carrying tales of exploding stars, colliding galaxies, and the relentless dance of black holes. They are the messengers from the most extreme corners of our universe, whispering secrets of unfathomable energy and unimaginable phenomena.
So, there you have it, the realm of high-energy waves – a testament to the profound interplay between wavelength and energy. These extraordinary waves are the architects of groundbreaking medical advancements, industrial breakthroughs, and cosmic discoveries. They remind us of the intricate tapestry of the electromagnetic spectrum and the hidden forces that shape our world and beyond.
Low Energy and Long Wavelengths: Exploring the Subtle Realm of Electromagnetic Waves
In the vast tapestry of electromagnetic waves, those with low energy possess a unique characteristic: they have long wavelengths. This fundamental relationship paints a fascinating picture of the quantum nature of light and its diverse manifestations in the world around us.
Radio Waves and Microwaves: The Gentle Giants
Radio waves and microwaves epitomize the realm of low-energy waves. Their wavelengths can stretch from centimeters to meters, enabling them to bypass obstacles easily. These gentle giants find myriad applications, from wireless communication to radar technology.
Radio waves, with their ability to travel long distances without significant attenuation, form the backbone of global communication networks. They carry voices, data, and music across continents, connecting people and fostering global connectivity.
Microwaves, renowned for their ability to penetrate food, are indispensable in our kitchens. Microwave ovens use electromagnetic energy to excite water molecules, generating heat that cooks food from within. These waves also find use in radar systems, where they bounce off objects to determine their location and speed.
The Spectrum and Its Wonders
The electromagnetic spectrum is a captivating symphony of electromagnetic waves, each with its unique energy and wavelength. From high-energy gamma rays to low-energy radio waves, the spectrum paints a vibrant portrait of the diverse nature of light.
In this spectrum, low-energy waves occupy the lower end, characterized by their long wavelengths. Radio waves, with their seemingly infinite reach, reside at the very bottom, followed by microwaves, infrared radiation, and the visible light spectrum.
The relationship between wavelength and energy is a cornerstone of understanding light and electromagnetic waves. Low-energy waves, with their long wavelengths, play a vital role in our daily lives, enabling communication, cooking, and much more. From the gentle hum of radio waves to the warmth of microwaves, these waves shape our world in myriad subtle yet profound ways.
Visible Light Spectrum:
- Define the visible light spectrum and its range of wavelengths.
- Explain how the human eye perceives different wavelengths of light.
The Fascinating Relationship Between Wavelength and Energy: A Journey Through the Electromagnetic Spectrum
Wavelength and energy are fundamental concepts in understanding light and electromagnetic waves. They are intimately connected, influencing each other in intriguing ways. Join us on a journey to explore this relationship and unravel the secrets of the electromagnetic spectrum.
The wavelength of a wave measures the distance between its successive crests or troughs. Conversely, energy represents the capacity of a wave to do work. Imagine an ocean wave: a long wavelength wave has lower energy, while a short wavelength wave packs more energy.
This inverse proportional relationship between wavelength and energy is captured by Planck’s constant:
E = h * f = h * c / λ
where:
- E is energy
- h is Planck’s constant
- f is frequency
- c is the speed of light
- λ is wavelength
The formula reveals that shorter wavelengths correspond to higher energies, while longer wavelengths indicate lower energies.
This relationship is further intertwined with the wave-particle duality of light, which states that light behaves as both a wave and a particle. When light interacts with matter, it behaves like a particle named a photon. Each photon carries a specific amount of energy, quantized into discrete units.
The energy of a photon is directly proportional to its frequency, and inversely proportional to its wavelength:
E = h * f = h * c / λ
High-energy waves like gamma rays and X-rays have short wavelengths and carry immense energy, capable of penetrating matter with ease. In contrast, low-energy waves such as radio waves and microwaves have long wavelengths and possess less energy, making them widely used in communication and heating applications.
Our eyes are sensitive to a narrow range of wavelengths known as the visible light spectrum. This spectrum spans from red (long wavelength) to violet (short wavelength). We perceive different colors depending on the wavelength of light that reaches our retinas.
The relationship between wavelength and energy extends beyond visible light. The electromagnetic spectrum encompasses the entire range of electromagnetic waves, from long-wavelength radio waves to high-energy gamma rays. Each region of the spectrum has unique properties and applications.
In conclusion, the relationship between wavelength and energy is crucial for understanding light and the electromagnetic spectrum. This inverse proportion and the quantization of energy in waves shape our experience of the world, from the colors we see to the technologies we rely on.
The Quantum World of Electromagnetic Waves
In the realm of light and energy, a profound dance unfolds where wavelength and energy engage in an intricate waltz. From the towering heights of gamma rays to the gentle ebb and flow of radio waves, this cosmic symphony reverberates through our universe.
At the heart of this dance lies a fundamental truth: wavelength and energy are inversely proportional. The shorter the wavelength, the higher the energy, and vice versa. This relationship is elegantly expressed by Planck’s constant, a pivotal constant in quantum physics:
Energy = Planck's constant * Frequency
The frequency of a wave, measured in hertz (Hz), represents the number of oscillations or cycles it completes per second. Remarkably, frequency and wavelength are also inversely proportional. As frequency increases, wavelength decreases, further reinforcing the inverse relationship between wavelength and energy.
This enigmatic dance between wavelength and energy extends beyond the classical realm, delving into the quantum world. Here, light exhibits a wave-particle duality, behaving simultaneously as both a wave and a particle. The energy of light, in this quantum world, is not continuous but rather exists in discrete packets called photons.
Photons possess a specific amount of energy, with higher-energy photons corresponding to shorter wavelengths and lower-energy photons to longer wavelengths. This quantization of energy is a fundamental characteristic of electromagnetic waves, distinguishing them from their classical counterparts.
The electromagnetic spectrum is a vast tapestry of electromagnetic waves, ranging from the shortest-wavelength, highest-energy gamma rays to the longest-wavelength, lowest-energy radio waves. This spectrum encapsulates the full breadth of light’s manifestations, from the visible light we perceive with our eyes to the invisible forces that shape our world.
In the visible light spectrum, different wavelengths correspond to different colors, with violet having the shortest wavelength and red having the longest. Our eyes have evolved to discern this spectrum, allowing us to experience the vibrant hues that surround us.
As we traverse the electromagnetic spectrum, we encounter a fascinating array of phenomena. Gamma rays, with their penetrating power, have applications in medical imaging and cancer treatment. X-rays, with their shorter wavelengths, are used for diagnostic imaging and materials analysis. Ultraviolet rays, while invisible to our eyes, play a crucial role in vitamin D synthesis and can cause sunburn.
Microwaves, on the other hand, are used for heating food and communication, while radio waves are essential for wireless communication, navigation, and remote sensing. Each region of the electromagnetic spectrum has its distinct properties and applications, underpinning our technological advancements.
In conclusion, the relationship between wavelength and energy is a cornerstone of our understanding of light and electromagnetic waves. From the grandeur of cosmic gamma rays to the mundane comfort of microwaves, this intricate dance shapes our world in profound and remarkable ways.
Wavelength and Energy: A Tale of Inverse Proportionality
In the realm of light and electromagnetism, the dance between wavelength and energy is a captivating spectacle. Wavelength, measured in nanometers, represents the distance between consecutive peaks of an electromagnetic wave. Energy, on the other hand, quantifies the ability of a wave to do work, measured in electron volts or joules.
The relationship between these fundamental properties is a tale of inverse proportionality. As the wavelength of an electromagnetic wave decreases, its energy soars. This inverse relationship is captured by Planck’s constant, denoted by the letter h, which serves as a universal conversion factor between wavelength and energy.

Wave-Particle Duality: A Quantum Twist
Light, as we know it, possesses a paradoxical nature. It behaves both like a wave and a particle, a duality that has captivated physicists for centuries. In its particle form, light manifests as photons, discrete bundles of energy that carry the quantum of light energy. The energy of a photon is directly proportional to the frequency of the electromagnetic wave, which in turn is inversely proportional to the wavelength.
This photon energy equation, E = hf, highlights the quantization of energy in electromagnetic waves. Energy is not a continuous spectrum but rather exists in discrete packets.
The Electromagnetic Spectrum: A Symphony of Radiant Energy
The electromagnetic spectrum, a vast expanse of wavelengths and energies, encompasses the entire range of electromagnetic radiation. From the high-energy gamma rays, with their penetrating power, to the long-wavelength radio waves, used for communication, the electromagnetic spectrum weaves together a tapestry of phenomena.
Each region of the spectrum finds unique applications in various scientific and technological fields. X-rays, for instance, provide valuable insights into medical diagnostics, while microwaves power our culinary adventures. The spectrum is a symphony of radiant energy, each note resonating with a distinct purpose.
The relationship between wavelength and energy is a fundamental concept that underpins our understanding of light and electromagnetic waves. Their inverse proportionality governs the quantum nature of light and shapes the electromagnetic spectrum, allowing us to unravel the mysteries of the physical world. From high-energy gamma rays to low-energy radio waves, this relationship weaves the fabric of our scientific endeavors and technological advancements.