Understanding Hydronium Ions: Key To Unlocking The Acidity Of Aqueous Solutions
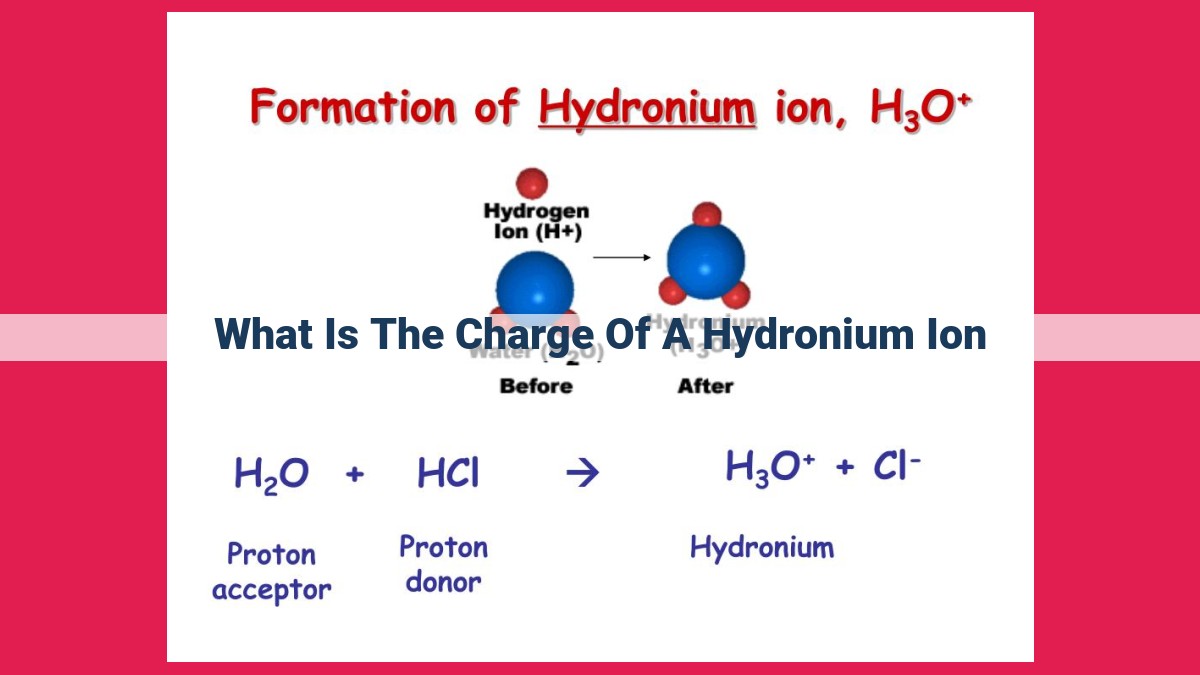
A hydronium ion (H3O+) forms when a hydrogen ion (H+) combines with a water molecule (H2O). The hydrogen ion carries a positive charge of +1, while the water molecule is neutral. Thus, the hydronium ion also has a net positive charge of +1. This positive charge plays a crucial role in determining the acidity of a solution, as hydronium ions are responsible for the acidic properties of water and other aqueous solutions.
In the vast realm of chemistry, we encounter various charged particles called ions. One such crucial ion is the hydronium ion, a key player in numerous chemical reactions. Join us on a fascinating journey to unravel the nature, formation, and significance of hydronium ions.
What is a Hydronium Ion?
Picture a hydrogen atom that has lost its precious electron, leaving it with a positive charge. This lone, wandering proton reluctantly forms a bond with a water molecule, creating a unique entity known as a hydronium ion, denoted by the symbol H3O+. Hydronium ions possess a positive charge, making them cations (positively charged ions).
Formation of Hydronium Ions
The creation of hydronium ions occurs through a delicate dance between hydrogen ions (H+) and water molecules (H2O). When these two entities come into contact, a chemical reaction takes place, resulting in the formation of a hydronium ion:
H+ + H2O → H3O+
This reaction is governed by the ionization constant of water (Kw), which represents the equilibrium constant for the dissociation of water into H+ and OH- ions.
Acidity and pH
Hydronium ions play a crucial role in determining the acidity of a solution. The higher the concentration of hydronium ions, the more acidic the solution. Chemists use a convenient scale called the pH scale to measure the acidity of solutions, with pH values ranging from 0 to 14. A pH of 7 indicates a neutral solution, while values below 7 denote acidic solutions and values above 7 indicate basic solutions.
Formation of Hydronium Ions
Imagine a dance between atoms and molecules, a fascinating chemical reaction where ions come to play. Let’s focus on a particularly important ion in chemistry: the hydronium ion.
The formation of hydronium ions begins with a hydrogen ion (H+) – a restless, “positively charged” particle. This hydrogen ion seeks a partner to share its positive vibe. And who better to dance with than a water molecule (H2O), which has a fun-loving “negative” side?
As the hydrogen ion approaches the water molecule, it finds a welcoming pocket where an oxygen atom forms a special bond with it. Voilà! A new dance partner is born: the hydronium ion (H3O+). This hydronium ion is a “cation” – a positively charged ion, ready to make its mark in chemical reactions.
Now, here’s the clever part: the formation of hydronium ions is not a one-time event. It’s a continuous dance, a constant exchange of hydrogen ions between water molecules. This dance is regulated by a special value called the “ionization constant of water.” This constant, represented by Kw, tells us the extent to which water molecules split into hydrogen ions and hydroxide ions (OH-).
The smaller the Kw value, the less likely water molecules are to split apart. This means fewer hydronium ions are formed, making the water solution less acidic. Conversely, a larger Kw value indicates a more acidic solution, with more hydronium ions breaking free.
So, there you have it – the formation of hydronium ions is a dynamic process, influenced by the dance between hydrogen ions and water molecules, governed by the ionization constant of water. It’s a fundamental step in understanding the acidity and pH of chemical solutions.
Acidity and pH: Understanding the Role of Hydronium Ions
In the realm of chemistry, acidity refers to a solution’s ability to release hydronium ions, positively charged ions composed of hydrogen atoms. These ions are the driving force behind many chemical reactions, and their concentration plays a crucial role in determining a solution’s acidity.
To quantify acidity, scientists use a logarithmic scale known as pH. This scale measures the concentration of hydronium ions in a solution, ranging from 0 to 14. The lower the pH, the higher the hydronium ion concentration, and the more acidic the solution becomes. Conversely, the higher the pH, the lower the hydronium ion concentration, indicating a more basic solution.
The pH scale is a valuable tool for chemists as it allows them to easily compare the acidity and basicity of different solutions. It also helps in understanding how certain reactions proceed and how to control their outcomes. For instance, in acid-base reactions, the strength of the acid or base can be predicted based on their pH values.
In summary, understanding the relationship between hydronium ion concentration and pH is essential for grasping the fundamentals of chemistry. By harnessing the power of the pH scale, scientists can decipher the intricate workings of chemical reactions and delve deeper into the fascinating world of acids and bases.
Electrical Charge and Ions: Uncovering the Secrets of Chemical Compounds
In the realm of chemistry, the concept of electrical charge plays a crucial role in understanding the behavior of substances. Atoms and molecules possess either a neutral, positive, or negative charge, influencing their chemical interactions.
Cations and anions are two key players in the world of charged particles. Cations are positively charged ions, while anions carry a negative charge. This charge difference arises from the gain or loss of electrons, the fundamental building blocks of atoms.
Hydrogen ions, abbreviated as H+, are the simplest cations, each carrying a single positive charge. They result from the loss of an electron by a hydrogen atom. On the flip side, hydroxide ions, denoted as OH-, are anions with a negative charge acquired by gaining an extra electron.
The interplay between hydrogen and hydroxide ions is vital in determining the acidity or basicity of a solution. A higher concentration of hydrogen ions indicates a more acidic solution, while a higher concentration of hydroxide ions signifies a more basic solution.
Understanding the electrical charge and the behavior of ions is fundamental to comprehending chemical reactions and their significance in various fields, including biology, medicine, and environmental science.
Ionization and the Hydronium Ion
Understanding Ionization
Ionization is a pivotal process in chemistry that involves the gaining or losing of electrons by atoms or molecules. This transformation results in the formation of charged particles known as ions. When an atom or molecule loses an electron, it becomes positively charged and forms a cation. Conversely, if it gains an electron, it becomes negatively charged and forms an anion.
In the realm of hydronium ions, the process of ionization plays a crucial role. A hydronium ion is a positively charged ion formed when a hydrogen ion (H+) combines with a water molecule (H2O).
The Formation of Hydronium Ions
The formation of hydronium ions occurs through ionization. When a hydrogen ion encounters a water molecule, the hydrogen ion donates its electron to the water molecule. This electron transfer results in the formation of a hydrogen atom (H) and a hydroxide ion (OH-). The hydrogen atom then combines with another water molecule to form a hydronium ion (H3O+).
The Charge of a Hydronium Ion
The charge of a hydronium ion stems from the loss of an electron by the hydrogen ion. Hydrogen ions carry a single positive charge (+1), and this charge is transferred to the hydronium ion. Therefore, the net charge of a hydronium ion is also +1.
Determining the Charge of a Hydronium Ion
Understanding the charge of a hydronium ion is crucial for comprehending its role in chemical reactions and acid-base chemistry. Let’s delve into the concept with a storytelling approach, breaking down the key factors that contribute to its charge.
As we know, a hydronium ion is formed when a hydrogen ion (H+) combines with a water molecule (H2O). This reaction results in the transfer of charge, creating a new chemical species with distinct properties.
The hydrogen ion contributes a positive (+1) charge to the hydronium ion. This is because a hydrogen ion is simply a proton, which lacks an electron. The absence of electrons gives it a positive charge.
Now, let’s consider the water molecule. When a hydrogen ion combines with water, the molecule undergoes a structural change, forming a hydronium ion (H3O+). This ionization process involves the loss of an electron from the hydrogen ion, which makes it positively charged.
Therefore, the net charge of a hydronium ion is determined by the sum of the charges contributed by the hydrogen ion and the water molecule. Since the hydrogen ion contributes a +1 charge and the water molecule contributes a zero charge (neutral), the overall charge of the hydronium ion is +1.
In summary, the charge of a hydronium ion is positive because it contains a positively charged hydrogen ion. This positive charge plays a significant role in the chemical reactions and acid-base properties of solutions.