Understanding Heat Capacity And Specific Heat: Quantifying Thermal Properties
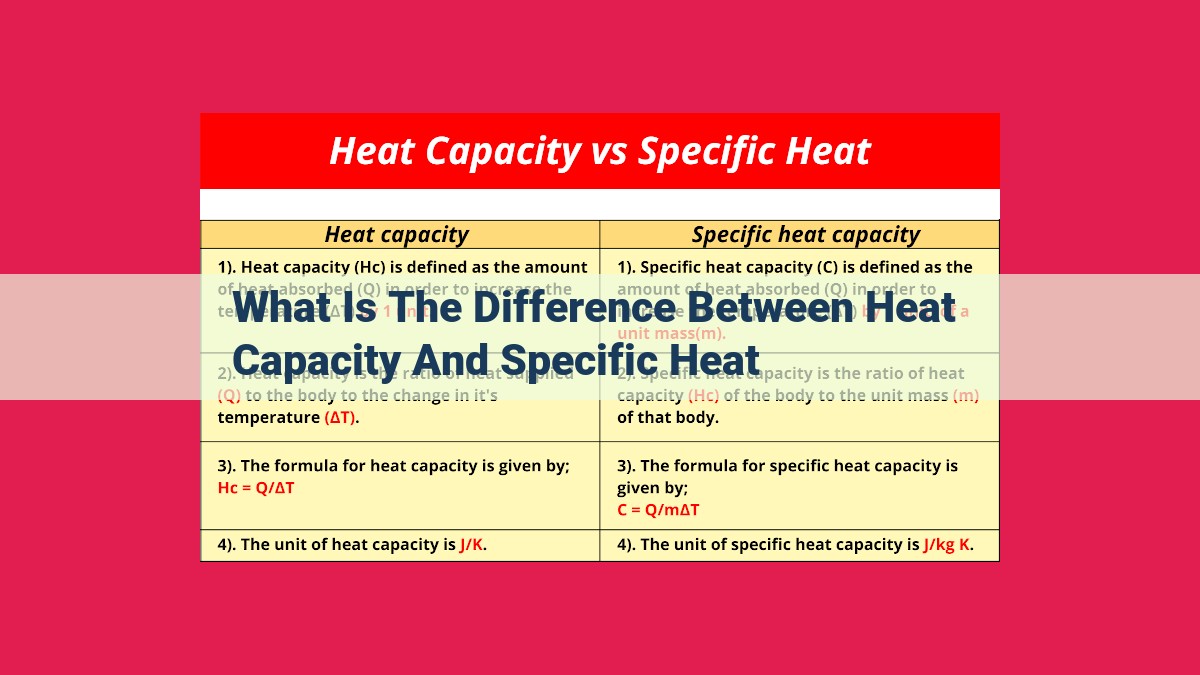
Heat capacity quantifies the total amount of heat required to raise the temperature of an object, while specific heat describes the amount of heat needed to raise the temperature of a unit mass of a substance by one temperature unit. Heat capacity depends on both mass and substance composition, whereas specific heat is a substance-specific property. The two are related by the equation: heat capacity = mass x specific heat. Heat capacity indicates an object’s ability to store heat, while specific heat determines the ease with which a substance’s temperature changes when heated.
Understanding Heat Capacity and Specific Heat
In the realm of thermodynamics, heat capacity and specific heat are two crucial concepts that delve into the intricate relationship between heat and matter. While often used interchangeably, these terms hold distinct meanings that unravel the mysteries of heat exchange.
Heat Capacity: The Heat-Soaking Sponge
Imagine a massive vessel filled with water. When heat is applied, the water doesn’t heat up as quickly as a smaller cup of the same liquid. This is because the heat capacity of an object refers to its ability to absorb heat without experiencing a significant temperature change. It’s like the heat’s favorite sponge, soaking up the warmth but holding it within its embrace. Heat capacity depends not only on the object’s mass but also on the substance it’s made of.
Specific Heat: The Substance-Selective Heat-Seeker
Unlike heat capacity, specific heat is a substance-specific property that describes how much heat is required to raise the temperature of one gram of that substance by one degree Celsius. It’s like the substance’s unique fingerprint, determining how efficiently it can absorb and store heat. Specific heat varies from substance to substance, with water being a renowned heat-seeker.
Bridging the Divide: The Mathematical Connection
Heat capacity and specific heat are interconnected through a simple equation:
Heat Capacity = Mass × Specific Heat
This equation highlights how these two concepts work together to capture the heat-storing and substance-specific nature of matter.
Mass Dependence: Size Matters
The heat capacity of an object is directly proportional to its mass. The more massive the object, the more heat it can absorb. However, specific heat remains constant for a given substance, regardless of the object’s size.
Substance Dependence: The Substance’s Signature
The specific heat of a substance depends on its molecular structure and chemical composition. Different substances have unique abilities to absorb and store heat, making specific heat a valuable tool for material scientists and engineers.
In conclusion, heat capacity and specific heat are essential concepts for understanding thermal behavior and designing efficient systems. From thermal engineering to material science, these concepts play a crucial role in shaping our technological advancements.
Heat Capacity: The Heat-Storing Ability of Objects
In the realm of thermal energy, understanding the concept of heat capacity is crucial. It signifies the amount of heat an object can absorb or release without undergoing any significant change in its temperature. Think of it as the object’s innate ability to store heat.
The heat capacity of an object depends on two primary factors: mass and the substance composition.
-
Mass Dependence: The larger the mass of an object, the greater its heat capacity. This means that it can absorb or release more heat without a substantial temperature change. Imagine two pots of water of different sizes; the larger pot has a higher heat capacity and can hold more heat before its temperature rises.
-
Substance Dependence: Different substances have distinct heat capacities. Water, for instance, has a relatively high heat capacity compared to metals like aluminum. This means that water can absorb or release more heat per unit mass than aluminum before showing a noticeable temperature shift.
Understanding the heat capacity of various substances is essential in various fields, such as thermal engineering and material science. It plays a pivotal role in designing efficient heating and cooling systems, selecting appropriate materials for specific applications, and comprehending heat transfer phenomena.
By grasping the heat capacity of objects, scientists and engineers can optimize energy consumption, enhance thermal performance, and innovate new thermal technologies that shape our modern world.
Specific Heat: The Substance-Specific Heat Requirement
As we delve deeper into the realm of thermal properties, we encounter the concept of specific heat, a crucial factor in understanding how different substances interact with heat. Unlike heat capacity, which measures the total amount of heat required to raise the temperature of an entire object, specific heat focuses on the per-unit mass requirement.
In essence, specific heat represents the amount of heat needed to elevate the temperature of one gram of a substance by one degree Celsius. This intrinsic property varies greatly across different materials, reflecting the unique intermolecular forces and atomic arrangements that govern each substance’s behavior.
Key Distinctions from Heat Capacity:
While both heat capacity and specific heat deal with heat and temperature changes, they hold distinct meanings. Heat capacity is an extensive property, meaning it depends on the mass of the object, while specific heat is an intensive property, independent of mass and solely determined by the substance.
Applications in Engineering and Science:
The concept of specific heat plays a pivotal role in various scientific and engineering fields:
-
In thermal engineering, it aids in designing efficient heat exchangers and predicting the temperature variations of fluids.
-
In material science, it helps determine a substance’s ability to store and release heat, influencing factors like thermal insulation and energy storage.
Understanding specific heat empowers us to comprehend how different substances respond to heat, enabling us to design and optimize systems that harness or mitigate thermal energy effectively.
Units and Relationship: Connecting Heat Capacity and Specific Heat
In the realm of thermodynamics, understanding the relationship between heat capacity and specific heat is crucial. These concepts help us quantify the ability of substances to absorb and store thermal energy.
Heat Capacity Units: The Thermal Reservoir
Heat capacity measures the total amount of heat required to raise the temperature of an object by one degree Celsius or Kelvin. Its units are expressed in Joules per degree Celsius (J/°C) or Joules per Kelvin (J/K). Heat capacity is a fundamental characteristic of an object, like its mass or volume.
Specific Heat: The Substance-Specific Requirement
In contrast, specific heat quantifies the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius or Kelvin. Unlike heat capacity, specific heat is a substance-specific property, meaning it varies depending on the material. Its units are Joules per gram per degree Celsius (J/g/°C) or Joules per gram per Kelvin (J/g/K).
The Equation That Unites Them
A crucial equation connects heat capacity (C) with specific heat (c) and mass (m):
Heat Capacity (_C_) = Specific Heat (_c_) x Mass (_m_)
This equation allows us to convert between heat capacity and specific heat for a given mass of substance. By knowing the specific heat of a material, we can easily calculate its heat capacity and vice versa.
Example: Water and Aluminum
Water has a specific heat of 4.18 J/g/°C, meaning it takes 4.18 Joules of heat to raise the temperature of one gram of water by one degree Celsius. Aluminum, on the other hand, has a lower specific heat of 0.9 J/g/°C. This means that for the same mass, aluminum will require less heat to undergo the same temperature increase compared to water.
Mass Dependence: The Role of Object Size
In the realm of heat transfer, the size of an object plays a crucial role in determining its ability to store heat. This concept is encapsulated in the property known as heat capacity.
Heat capacity represents the amount of heat energy required to raise the temperature of an object by one degree Celsius. It is directly proportional to the mass of the object. In other words, the more massive an object, the more heat it can absorb without experiencing a significant temperature change.
This dependence on mass stems from the fact that heat energy is distributed throughout the material of an object. When heat is added to a more massive object, the energy becomes dispersed over a larger volume, resulting in a smaller temperature increase.
Conversely, specific heat, a measure of the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius, remains constant regardless of the mass of the object. This implies that the material’s composition, rather than its size, governs its ability to absorb heat per unit mass.
Substance Dependence: The Influence of Material Composition
When it comes to understanding how different materials behave when heated, the nature of their substance plays a crucial role. Both heat capacity and specific heat exhibit a strong relationship with the material composition of the object being heated.
Heat capacity, as we know, is a measure of the total amount of heat an object can absorb or release without experiencing a significant change in temperature. It depends not only on the mass of the object but also on the specific heat of the substance it is made of.
Specific heat, on the other hand, characterizes the heat-absorbing ability of the substance itself. It is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one unit of temperature.
The substance dependence of heat capacity and specific heat means that different materials require different amounts of heat to change their temperature by the same amount. For instance, metals typically have a higher specific heat compared to plastics, indicating that they require more heat energy to elevate their temperature.
This variability stems from the unique atomic and molecular structures of different substances. Materials with stronger intermolecular bonds generally have a lower specific heat, as more energy is needed to overcome the attractive forces between the molecules and increase their kinetic energy.
Understanding the substance dependence of heat capacity and specific heat is essential in various applications, particularly in fields such as thermal engineering and material science. It helps engineers design systems that can effectively handle the transfer and storage of heat, while also guiding the selection of materials that can withstand specific temperature ranges.
Applications: Heat Capacity and Specific Heat in Practice
The concepts of heat capacity and specific heat find widespread applications in various fields, including thermal engineering and material science. Understanding these properties is crucial for designing systems and materials that can efficiently manage heat transfer.
Thermal Engineering
Thermal engineers rely on heat capacity and specific heat to design systems that can withstand extreme temperatures or efficiently transfer heat. For instance, in power plants, heat capacity is used to calculate the amount of thermal energy stored in boilers, while specific heat helps determine the cooling requirements of condensers.
Material Science
Material scientists utilize heat capacity and specific heat to understand the thermal properties of different materials. This knowledge is vital in selecting materials for specific applications. For example, in the aerospace industry, materials with high specific heat are used to dissipate heat generated during flight.
Construction
In construction, heat capacity and specific heat are essential for designing energy-efficient buildings. Materials with high heat capacity, such as concrete, can absorb and release heat slowly, resulting in more stable indoor temperatures. Similarly, understanding specific heat helps architects determine the thermal insulation requirements of building materials.
Automotive Industry
The automotive industry uses heat capacity and specific heat to design efficient engines_ and cooling systems. High heat capacity materials in engine blocks help prevent overheating, while low specific heat coolants effectively transfer heat away from the engine. Additionally, specific heat is used to calculate the energy required to heat or cool a vehicle’s cabin.
Industrial Processes
In industrial processes, heat capacity and specific heat are crucial for optimizing energy consumption_. For example, in refineries, heat capacity is used to design furnaces and reactors to minimize heat loss. Similarly, in food processing, understanding specific heat hilft engineers optimize heating and cooling systems for preserving the quality of food products.