Understanding Gas Laws: Relationships Between Pressure, Volume, Temperature, And Moles
Pressure and temperature are related through the gas laws. Boyle’s Law shows an inverse relationship between pressure and volume, while Charles’s Law exhibits a direct relationship between volume and temperature. Gay-Lussac’s Law establishes a direct relationship between pressure and temperature. The Combined Gas Law integrates these laws, relating pressure, volume, and temperature. The Ideal Gas Law extends the gas laws, considering the number of moles. These laws demonstrate how these variables affect each other, providing insights into gas behavior and the changes they undergo under varying conditions.
Boyle’s Law: Unraveling the Inverse Relationship Between Pressure and Volume
In the realm of gases, Boyle’s Law stands out as a fundamental principle that elucidates the intricate connection between pressure and volume. This law, discovered by the renowned scientist Robert Boyle in the 17th century, provides a remarkable insight into the behavior of gases under varying conditions.
At the heart of Boyle’s Law lies the concept of an inverse relationship between pressure and volume. This means that as pressure increases, volume decreases, and vice versa. Imagine a balloon filled with air. When you squeeze the balloon, the pressure exerted on the air inside increases, causing the balloon to shrink in volume. Conversely, when you release the balloon, the pressure decreases, allowing the balloon to expand and increase in volume.
The inverse relationship between pressure and volume can be attributed to the kinetic energy of gas molecules. As pressure increases, the molecules are forced closer together, resulting in more frequent collisions with the container walls. This increased collision frequency, in turn, exerts a greater force on the walls, leading to an increase in pressure. Conversely, as volume increases, the molecules have more space to move, reducing the collision frequency and, consequently, the pressure.
Boyle’s Law is a powerful tool that allows scientists and engineers to predict the behavior of gases in various applications. It finds practical applications in fields such as scuba diving, where understanding the relationship between pressure and volume is crucial for ensuring the safety of divers at different depths. Additionally, Boyle’s Law plays a vital role in the design of gas storage systems, enabling engineers to optimize the storage capacity and pressure for various gases, such as hydrogen and natural gas.
Charles’s Law: A Direct Correlation Between Volume and Temperature
Unveiling the fascinating world of gases, Charles’s Law unveils a profound relationship between the volume and temperature of a gas at constant pressure. This fundamental law, discovered by the renowned physicist Jacques Alexandre Charles, provides a deeper understanding of the behavior of gases under varying conditions.
In the realm of constant pressure, Charles’s Law establishes a direct and proportional relationship between the volume of a gas and its absolute temperature (measured in Kelvin). As the temperature of a gas increases, its volume also increases, and vice versa. This phenomenon stems from the increased kinetic energy of gas molecules at higher temperatures, causing them to move more rapidly and occupy a larger space.
Perhaps the most intriguing aspect of Charles’s Law lies in its ability to predict the behavior of gases under changing temperature conditions. By utilizing the equation V/T = constant, we can determine the change in volume of a gas when its temperature changes. This equation implies that the ratio of volume (V) to temperature (T) remains constant for a given sample of gas at constant pressure.
For instance, if we have a gas sample with an initial volume of 100 mL at 273 K (0°C), and we increase the temperature to 373 K (100°C), Charles’s Law predicts that the volume will increase to approximately 136 mL. This increase is a direct consequence of the increased kinetic energy of the gas molecules at the higher temperature.
Understanding Charles’s Law is crucial for numerous applications in science and engineering. It plays a significant role in fields such as thermodynamics, meteorology, and even cooking. By harnessing the principles of Charles’s Law, scientists and engineers can predict the behavior of gases under various conditions, leading to advancements in technology and a deeper comprehension of the physical world.
Gay-Lussac’s Law: Unraveling the Pressure-Temperature Enigma
In the world of gases, one of the most intriguing relationships is the interplay between pressure and temperature. Gay-Lussac’s Law sheds light on this enigmatic connection, revealing a direct relationship between these two physical properties of gases.
Imagine a gas trapped within a container of constant volume. As you gradually increase the temperature of the gas, something peculiar happens: the pressure inside the container also rises proportionally. This phenomenon, as described by Gay-Lussac’s Law, suggests that the gas molecules become more energetic with increasing temperature.
This increased molecular energy translates into a greater force exerted by the gas molecules against the container walls. As the temperature soars, the molecules collide with the walls more frequently and with greater force, resulting in an upward trend in pressure.
The mathematical expression for Gay-Lussac’s Law is:
P/T = constant
where:
- P represents the pressure of the gas
- T represents the absolute temperature of the gas
This equation underscores the direct proportionality between pressure and absolute temperature. In essence, as the absolute temperature of a gas increases, the pressure of the gas also increases, assuming the volume remains constant.
This law is crucial in various scientific and industrial applications. For instance, it helps engineers understand the behavior of gases in enclosed systems, such as sealed containers or gas cylinders. By manipulating temperature and volume, they can control the pressure and, consequently, the behavior of the gas for specific purposes.
In conclusion, Gay-Lussac’s Law unveils the direct correlation between pressure and absolute temperature for gases at constant volume. This fundamental principle provides valuable insights into the behavior of gases and their applications in a wide range of fields.
The Combined Gas Law: A Unified Equation for Pressure, Volume, and Temperature
In the realm of gases, three fundamental laws govern their behavior: Boyle’s Law, Charles’s Law, and Gay-Lussac’s Law. Each describes a specific relationship between two of the variables: pressure (P), volume (V), and temperature (T). The combined gas law combines these laws into a single comprehensive equation, providing a powerful tool to understand gas behavior under varying conditions.
Boyle’s Law: Pressure and Volume
Imagine a gas confined in a cylinder with a movable piston. According to Boyle’s Law, when the temperature is kept constant, increasing the pressure will cause a proportional decrease in volume, and decreasing the pressure will increase the volume. This inverse relationship can be represented by the equation P₁V₁ = P₂V₂, where P₁ and V₁ are the initial pressure and volume, respectively, and P₂ and V₂ are the final values.
Charles’s Law: Volume and Temperature
Now, let’s consider a gas in a sealed container, meaning the volume cannot change. According to Charles’s Law, when the pressure is kept constant, increasing the temperature will cause a proportional increase in volume, and decreasing the temperature will decrease the volume. This direct relationship is expressed as V₁/T₁ = V₂/T₂, where T₁ and V₁ are the initial temperature and volume, and T₂ and V₂ are the final values.
Gay-Lussac’s Law: Pressure and Temperature
Finally, let’s explore a gas in a container with a fixed volume. According to Gay-Lussac’s Law, when the volume is kept constant, increasing the temperature will cause a proportional increase in pressure, and decreasing the temperature will decrease the pressure. This direct relationship can be written as P₁/T₁ = P₂/T₂, where P₁ and T₁ are the initial pressure and temperature, and P₂ and T₂ are the final values.
The Combined Gas Law: Integrating the Laws
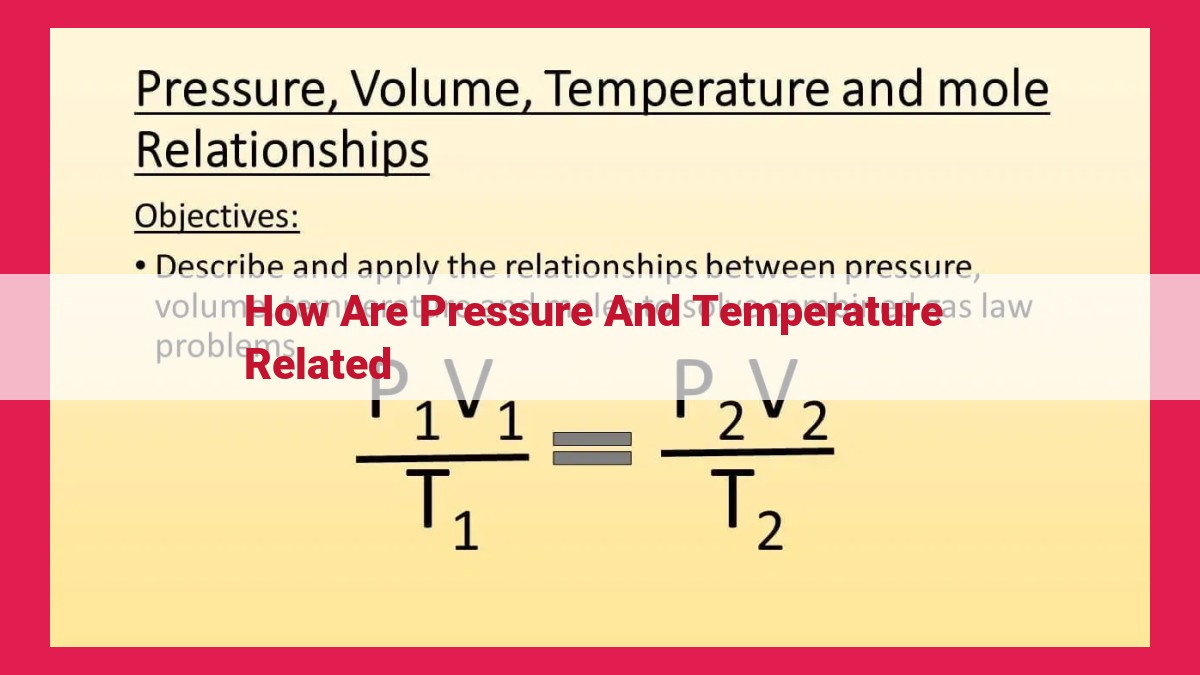
The combined gas law integrates all three laws into a single equation that relates pressure, volume, and temperature under varying conditions. It states that (P₁V₁)/T₁ = (P₂V₂)/T₂, where the subscripts 1 and 2 denote the initial and final states of the gas.
Applications of the Combined Gas Law
The combined gas law has wide-ranging applications in various fields. It is used to:
- Predict the changes in gas properties during reactions or processes
- Calculate the volume or pressure of a gas at different temperatures
- Analyze the behavior of gases in closed systems and in the atmosphere
By understanding the combined gas law, we gain a deeper insight into the behavior of gases and how they interact with their surroundings. It is a powerful tool that enhances our understanding of the physical world.
Extending the Gas Laws: The Ideal Gas Law
The ideal gas law is a more comprehensive equation that incorporates all the relationships established by Boyle’s, Charles’s, and Gay-Lussac’s laws. It extends these empirical observations to include the number of moles of a gas, allowing for more accurate predictions of gas behavior under various conditions.
The ideal gas law is expressed as:
PV = nRT
where:
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m³)
- n is the number of moles of gas in mol
- R is the ideal gas constant, which is 8.314 J/mol*K
- T is the temperature of the gas in kelvins (K)
This equation combines Boyle’s, Charles’s, and Gay-Lussac’s laws in a single comprehensive formula. By incorporating the number of moles, the ideal gas law provides a more accurate description of gas behavior, especially at high pressures and low temperatures. This law is particularly useful for calculating the properties of gases in various applications, such as predicting the volume of a gas at different temperatures or the pressure of a gas in a closed container.