Understanding Fermentation And Cellular Respiration: Key Differences
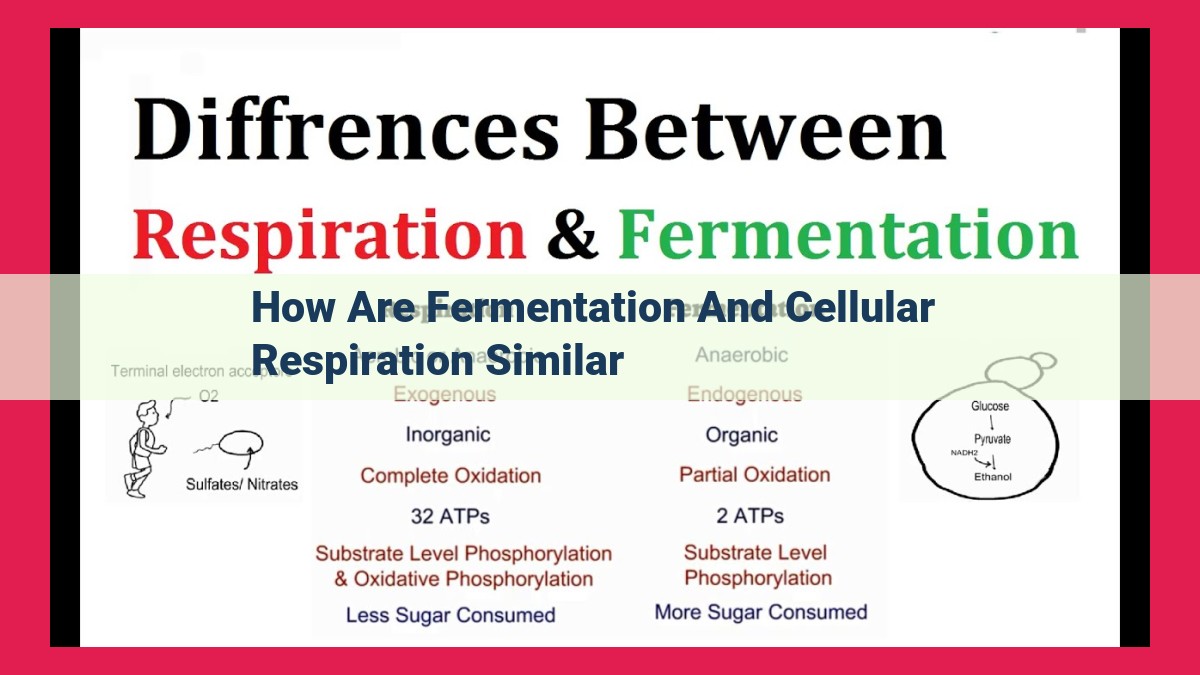
Fermentation and cellular respiration share fundamental similarities. Both processes utilize ATP as the primary energy currency, employing NADH and NADPH as electron carriers. Fermentation generates ATP through substrate-level phosphorylation, while cellular respiration oxidizes glucose entirely in the presence of oxygen, resulting in a higher ATP yield. Despite being anaerobic, fermentation provides an immediate energy source when oxygen is scarce, while cellular respiration ensures sustained energy production in aerobic conditions.
Similarities Between Fermentation and Cellular Respiration: An Unexpected Alliance in the Energy Factory
ATP: The Universal Energy Currency
The cellular powerhouses we call our cells have a secret weapon in their arsenal: ATP, the primary energy currency in both fermentation and cellular respiration. Think of it as the cash your cells use to buy stuff, like building new proteins or powering up your muscles. Fermentation and cellular respiration, two distinct metabolic pathways, both use ATP to fuel the cellular machinery.
Electron Carriers: NADH and NADPH – The Helpers
Imagine NADH and NADPH as the waiters in the cellular restaurant. When glucose, our body’s main energy source, is broken down, these waiters rush over to the electron bar and pick up electrons. They then deliver these electrons to the respiratory chain, a series of electron-hungry molecules. The flow of electrons along the respiratory chain, like a cascading waterfall, generates the energy needed to make ATP.
Substrate Level Phosphorylation – The Quick Energy Fix
Fermentation has a clever trick up its sleeve: substrate level phosphorylation. It’s a quick way to generate ATP without the need for a full-blown respiratory chain. Fermentation intercepts glucose during glycolysis and uses enzymes to directly “clip” phosphate groups onto ADP, creating ATP. Think of it as a shortcut to energy production, although this shortcut comes at a cost – limited ATP yield.
The Role of Oxygen – A Key Distinction
Here’s where fermentation and cellular respiration take different paths. Fermentation is an anaerobic process, meaning it doesn’t require oxygen. It’s like a resourceful party-thrower who can have a good time without music. Cellular respiration, on the other hand, is an aerobic process, like a diva that needs a spotlight. It relies on oxygen to fully oxidize glucose, squeezing every last drop of energy out of it and producing far more ATP than fermentation.
Efficiency: The Winner Takes All
When it comes to ATP production, cellular respiration reigns supreme. With its complete oxidation of glucose, it generates 32-34 molecules of ATP for every glucose molecule, while fermentation only produces a paltry 2. It’s like comparing a super-efficient solar panel to a dim flickering candle.
Interplay: Teamwork for Energy Success
Despite their differences, fermentation and cellular respiration are not rivals but partners. Fermentation provides a quick energy boost in the absence of oxygen, like a backup generator during a power outage. Cellular respiration takes over when oxygen is available, sustaining long-term energy production. Together, they form a dynamic duo, ensuring that our cells never run out of fuel.
Similarities Between Fermentation and Cellular Respiration: A Tale of Energy Currency and Efficiency
In the bustling world of cellular energy metabolism, two fundamental pathways reign supreme: fermentation and cellular respiration. While distinct in their execution, these processes share striking similarities that underpin their vital role in sustaining life.
ATP: The Fuel of Cellular Activity
At the heart of both fermentation and cellular respiration lies ATP (adenosine triphosphate). This molecule is the body’s primary energy currency, a miniature battery that powers cellular machinery. Like a rechargeable battery, ATP releases energy by breaking apart its chemical bonds. This energy fuels a myriad of cellular activities, including muscle contractions, nerve impulses, and chemical synthesis.
Electron Carriers: NADH and NADPH
NADH and NADPH (nicotinamide adenine dinucleotide and its phosphorylated form) serve as electron carriers, shuttling electrons from substrate molecules during glycolysis and the citric acid cycle. These electrons are essential for generating ATP in both fermentation and cellular respiration. They act as the mediators, enabling the transfer of energy from nutrient molecules to the energy-storing powerhouse of ATP.
**Fermentation and Cellular Respiration: Two Sides of the Energy Coin**
Electron Carriers: NADH and NADPH
In the intricate world of cellular energy production, two indispensable electron carriers play a pivotal role: NADH and NADPH. Imagine these molecules as tiny rechargeable batteries that accept electrons during key metabolic reactions, providing the fuel for subsequent energy production.
NADH (nicotinamide adenine dinucleotide) and NADPH (nicotinamide adenine dinucleotide phosphate) share a similar structure but have distinct functions in different cellular processes. Both molecules contain a nicotinamide ring that undergoes reversible oxidation-reduction reactions, allowing them to accept and release electrons.
During glycolysis, the first stage of both fermentation and cellular respiration, NADH is generated. When glucose is broken down into smaller molecules, it transfers electrons to NAD+, which in turn becomes NADH. NADH carries these electrons to the electron transport chain, a series of protein complexes in the inner mitochondrial membrane. Here, the electrons are passed down the chain, releasing energy used to pump protons across the membrane, creating a proton gradient that ultimately powers ATP synthesis.
NADPH, on the other hand, is primarily generated during the pentose phosphate pathway. This pathway plays a crucial role in producing nucleotides and other essential biomolecules. Unlike NADH, which feeds electrons into the electron transport chain for ATP production, NADPH donates electrons to reduce various substrates, including those involved in detoxification, DNA synthesis, and fatty acid synthesis.
Unraveling the Similarities Between Fermentation and Cellular Respiration: A Tale of Energy and Efficiency
In the intricate tapestry of life, energy plays a pivotal role, fueling every cellular function. Two fundamental processes, fermentation and cellular respiration, share remarkable similarities in their quest to generate this vital energy.
NADH and NADPH: The Electron Carriers
Imagine the bustling streets of a city, teeming with electrons eager to deliver their energy. Just as cars carry people, two electron carriers, NADH and NADPH, transport these energy-rich electrons through metabolic pathways.
During the initial stages of both fermentation and cellular respiration, glycolysis, electrons are captured from glucose molecules and tucked away into NADH and NADPH. Like vigilant guardians, these carriers safeguard these electrons, ready to release them at the appropriate moment.
As the metabolic journey continues, the citric acid cycle, a labyrinth of chemical reactions, further enriches the bounty of electrons. NADH and NADPH greedily accept these additional electrons, becoming even more potent energy reservoirs.
Interplay of Energy Pathways: A Dynamic Duo
Fermentation and cellular respiration embark on distinct paths, yet they share a common destination: providing energy for cellular processes. Fermentation, like a sprinter, offers a quick burst of energy when oxygen is scarce. It generates a modest amount of ATP, the universal energy currency, through a process called substrate-level phosphorylation.
Cellular respiration, in contrast, resembles a marathon runner, delivering sustained energy over a longer duration. This is because it utilizes the complete oxidation of glucose, extracting far more energy and generating a greater yield of ATP than fermentation.
Efficiency: The Key to Endurance
The efficiency of an energy-generating process refers to the amount of energy it extracts from its fuel. Cellular respiration emerges as the clear winner in this regard, boasting an impressive efficiency of around 36-38%. This remarkable efficiency stems from its full exploitation of glucose’s energy potential.
In contrast, fermentation, with its reliance on partial oxidation, achieves an efficiency of only about 2-4%. Despite its lower efficiency, fermentation plays a crucial role when oxygen levels are low, ensuring a continuous supply of energy for cellular activities.
Unlocking the Secrets of Fermentation and Cellular Respiration: A Biological Saga
In the depths of every living cell, two metabolic pathways wage a silent battle for energy dominance: fermentation and cellular respiration. While these processes share intriguing similarities, their differences hold the key to understanding how cells survive and thrive.
The Energy Currency: ATP
Both fermentation and cellular respiration rely heavily on adenosine triphosphate (ATP), the universal energy currency of life. ATP acts like a microscopic rechargeable battery, providing energy for a vast array of cellular functions.
Electron Carriers: NADH and NADPH
Like busy messengers, nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH) flit about the cell, transporting electrons like precious gems. These electron carriers capture electrons from glucose during glycolysis and the citric acid cycle.
Substrate Level Phosphorylation: The Quick Energy Fix
In fermentation, a process that can occur in the absence of oxygen, a unique mechanism called substrate level phosphorylation generates a limited amount of ATP. This process is analogous to a crafty thief pilfering a little energy from glucose without fully breaking it down.
The Role of Oxygen: From Anaerobic to Aerobic
Fermentation is an anaerobic process, meaning it doesn’t require oxygen. This makes it ideal for cells that live in oxygen-deprived environments, such as muscle cells during intense exercise or bacteria thriving in the gut.
Cellular respiration, on the other hand, is a more complex and aerobic process, devouring oxygen as it efficiently breaks down glucose. This oxygen-dependent pathway yields a far greater bounty of ATP than fermentation.
Efficiency: A Tale of Two Pathways
Cellular respiration stands as the undisputed champion of energy efficiency. By fully oxidizing glucose, it generates a staggering 36-38 molecules of ATP. Fermentation, in contrast, produces a mere 2 molecules of ATP, a testament to its quick-fix nature.
The Interplay of Fermentation and Cellular Respiration: A Dynamic Duo
These metabolic pathways are not solitary actors but rather a dynamic duo. Fermentation provides an immediate burst of energy when oxygen is scarce, while cellular respiration takes over when oxygen becomes available, sustaining long-term energy production.
Their combined efforts ensure that cells can adapt to changing conditions, harnessing energy from glucose to fuel their vital functions.
Similarities Between Fermentation and Cellular Respiration: Unveiling the Hidden Connection
Fermentation and cellular respiration, two vital metabolic pathways, share striking similarities despite their differences. Like cogs in a well-oiled machine, they interplay to generate ATP, the ubiquitous energy currency that fuels myriad cellular processes.
Substrate Level Phosphorylation: A Direct Approach to Energy Production
During fermentation, a process that occurs in the absence of oxygen, substrate level phosphorylation plays a crucial role. This process involves the direct transfer of a phosphate group from a substrate molecule, primarily glucose, to ADP, yielding ATP. While straightforward and efficient, substrate level phosphorylation generates a limited quantity of ATP, typically only 2 molecules per glucose molecule.
The reason for this energy limitation lies in the nature of fermentation. Unlike cellular respiration, which harnesses the oxygen molecule as a final electron acceptor, fermentation relies on organic molecules, such as pyruvate, for this role. This organic acceptor has a limited capacity to accept electrons, resulting in a partial oxidation of glucose and, consequently, a more modest ATP yield.
Nonetheless, fermentation serves a vital purpose. It provides an immediate energy source when oxygen, the preferred electron acceptor, is scarce or unavailable. In anaerobic environments, such as muscle cells during intense exercise or certain microorganisms, fermentation steps in to meet the urgent energy demands of cells.
Fermentation vs. Cellular Respiration: Similarities and Differences
In the bustling metropolis of our bodies, energy is the lifeblood that powers every cellular process. Two essential metabolic pathways, fermentation and cellular respiration, are responsible for generating this vital currency. While they share some striking similarities, their distinct characteristics reveal a tale of adaptation and efficiency.
The Energy Currency: ATP
ATP (adenosine triphosphate) serves as the universal energy currency for all living organisms, including the cells within our bodies. Both fermentation and cellular respiration generate ATP, which provides the fuel for cellular activities like muscle contraction, protein synthesis, and nerve impulses.
Electron Carriers: NADH and NADPH
Like messengers carrying energy, NADH (nicotinamide adenine dinucleotide) and NADPH (nicotinamide adenine dinucleotide phosphate) play crucial roles in both pathways. These electron carriers capture electrons from substrate molecules during glucose breakdown.
Substrate Level Phosphorylation
Fermentation utilizes a process called substrate level phosphorylation to create a modest amount of ATP. This process directly transfers high-energy phosphate groups from substrate molecules to ADP (adenosine diphosphate), resulting in the formation of ATP.
The Role of Oxygen
Fermentation unfolds as an anaerobic process, occurring in the absence of oxygen. Cellular respiration, on the other hand, thrives as an aerobic pathway, requiring oxygen to complete its metabolic cycle.
Efficiency: A Tale of Two Pathways
Fermentation falls short in terms of energy efficiency compared to cellular respiration. This discrepancy stems from the fact that fermentation only partially oxidizes glucose, yielding a relatively small amount of ATP (2 ATP molecules per glucose molecule). In contrast, cellular respiration fully oxidizes glucose, generating a far greater ATP yield (up to 38 ATP molecules per glucose molecule).
Interplay and Adaptation
Despite their differences, fermentation and cellular respiration work in harmony to fulfill the energy demands of our bodies. Fermentation serves as an immediate energy source when oxygen is scarce or unavailable, providing a backup plan for cellular respiration. Cellular respiration, however, takes over when oxygen becomes available, sustaining long-term energy production with its superior efficiency.
Explain how oxygen dependence affects the metabolic efficiency of each pathway.
Fermentation and Cellular Respiration: The Intertwined Dance of Energy Production
In the intricate ballet of cellular life, two metabolic pathways take center stage: fermentation and cellular respiration. These energy-producing processes share striking similarities, yet their subtle differences orchestrate a captivating tale of adaptation and efficiency.
One of their most fundamental commonalities lies in the precious currency of life: ATP. ATP, the adenosine triphosphate molecule, fuels myriad cellular activities. In both fermentation and cellular respiration, ATP plays a crucial role as the primary energy carrier. It acts like tiny rechargeable batteries, providing the power for cellular processes to thrive.
Another similarity lies in the vital electron carriers, NADH and NADPH. These molecules shuttle electrons, the energetic workhorses of metabolism, from substrate molecules during glycolysis and the citric acid cycle. They are the unsung heroes that drive the production of ATP.
However, a pivotal distinction emerges when we consider oxygen’s role in these pathways. Fermentation is an anaerobic process, meaning it occurs without oxygen. In contrast, cellular respiration is aerobic, relying on the presence of oxygen for its efficient energy production. This oxygen dependence profoundly influences the metabolic efficiency of each pathway.
The Intriguing Dance of Fermentation and Cellular Respiration: A Tale of Energy Currency and Metabolic Pathways
In the bustling metropolis of our cells, two energetic partners, fermentation and cellular respiration, tirelessly orchestrate the conversion of nutrients into the vital energy currency, ATP. As we delve into their intricate world, we shall unravel the striking similarities between these metabolic pathways.
Unveiling the Power of ATP
ATP, the cellular powerhouse, reigns supreme as the energy currency that fuels myriad cellular processes. Both fermentation and cellular respiration generate ATP through different mechanisms, ensuring a steady flow of energy throughout the cell.
Electron Shuttles: NADH and NADPH
Acting as electron carriers, NADH and NADPH play a pivotal role in both pathways. They diligently collect electrons from substrate molecules during glycolysis and the citric acid cycle, preparing them for the dance of ATP production.
Substrate Level Phosphorylation: A Fermenting Feature
In fermentation, a peculiar process known as substrate level phosphorylation takes center stage. This dance directly generates ATP from substrate molecules, albeit in limited quantities.
Oxygen: The Metabolic Switch
A striking distinction between these pathways lies in their dependence on oxygen. Fermentation flourishes in anaerobic environments, where oxygen is a scarce commodity. Cellular respiration, on the other hand, thrives in aerobic conditions, utilizing oxygen for its energy-maximizing potential.
Metabolic Efficiency: A Tale of Two Tales
Cellular respiration reigns supreme in energy efficiency, earning the title of the king of ATP production. Its ability to fully oxidize glucose unlocks a far greater ATP yield compared to fermentation.
Interplay of Energy Partners
These metabolic pathways are not adversaries but rather complementary partners. Fermentation serves as a rapid energy source when oxygen is scarce, while cellular respiration sustains long-term energy production in its presence. Together, they ensure an uninterrupted supply of ATP, fueling the vital functions of our cells and maintaining the delicate balance of life.
The Similarities and Differences Between Fermentation and Cellular Respiration
Fermentation and cellular respiration are two essential metabolic processes that provide energy for cells. While they share some similarities, there are also key differences that affect their efficiency and the amount of energy they produce.
Energy Currency: ATP
Both fermentation and cellular respiration rely on ATP as the primary energy currency. ATP is a molecule that stores energy in its chemical bonds and releases it when needed for cellular activities. Both processes use a series of chemical reactions to generate ATP from substrate molecules.
Electron Carriers: NADH and NADPH
Electron carriers, such as NADH and NADPH, play a crucial role in both pathways. These molecules accept electrons from substrate molecules during glycolysis and the citric acid cycle. The electrons are then transferred to the electron transport chain, which generates the proton gradient used to produce ATP.
Substrate Level Phosphorylation
Fermentation involves a process called substrate level phosphorylation. This process transfers a phosphate group from a substrate molecule directly to ADP, generating ATP. However, fermentation only generates a limited amount of ATP through this mechanism.
Role of Oxygen
A key difference between fermentation and cellular respiration is the presence or absence of oxygen. Fermentation is an anaerobic process, meaning it occurs in the absence of oxygen. Cellular respiration, on the other hand, is an aerobic process, meaning it requires oxygen.
Efficiency
Cellular respiration is more efficient than fermentation because it involves the complete oxidation of glucose. This means that glucose is completely broken down into carbon dioxide and water, releasing a significant amount of energy. Fermentation, on the other hand, only partially oxidizes glucose, resulting in the production of lactic acid or ethanol as byproducts.
Interplay of Fermentation and Cellular Respiration
Fermentation and cellular respiration often work together to provide energy for cells. Fermentation provides an immediate energy source in the absence of oxygen, while cellular respiration sustains long-term energy production when oxygen is available. This interplay ensures that cells have a continuous supply of energy to meet their metabolic needs.
Fermentation and Cellular Respiration: A Partnership for Cellular Energy
As cells embark on their life-sustaining adventures, they require a reliable source of energy to power their intricate machinery. Nature has bestowed upon them two remarkable pathways that fulfill this essential role: fermentation and cellular respiration. While they may differ in some aspects, these pathways share a common goal: to generate the cellular currency of energy, ATP.
The Energy Transformers: ATP and Electron Carriers
ATP (adenosine triphosphate) is the undisputed energy currency of cells. Resembling a rechargeable battery, ATP’s high-energy bonds release energy when broken, providing the fuel for cellular activities. Fermentation and cellular respiration are master energy transformers, converting glucose, the primary energy source, into ATP.
Electron carriers, such as NADH (nicotinamide adenine dinucleotide) and NADPH (nicotinamide adenine dinucleotide phosphate), play a crucial role in these energy conversions. They act as molecular taxis, shuttling electrons from glucose to the ATP-generating machinery.
Fermentation: A Quick Energy Fix
Fermentation, a clever energy-saving strategy, operates in the absence of oxygen. When oxygen is scarce, cells turn to fermentation to rapidly produce ATP. This process occurs within the cytoplasm, the cell’s bustling hub of activity.
Substrate-Level Phosphorylation
Fermentation generates ATP through a process called substrate-level phosphorylation. Enzymes directly transfer high-energy phosphate groups from glucose derivatives to ADP (adenosine diphosphate), creating ATP in a quick and efficient manner.
Cellular Respiration: The Powerhouse
Cellular respiration, in contrast, is an oxygen-dependent process that takes place in the mitochondria, the cell’s energy powerhouse. This pathway consists of three main stages: glycolysis, the citric acid cycle, and oxidative phosphorylation.
Oxidative Phosphorylation
Oxidative phosphorylation is the star performer of cellular respiration, generating most of the ATP. Oxygen, the key player in this process, serves as the final electron acceptor. As electrons flow down the electron transport chain, they release energy, which is used to pump protons across a membrane. The resulting proton gradient drives the synthesis of vast amounts of ATP.
The Symbiotic Dance of Fermentation and Cellular Respiration
Fermentation and cellular respiration work together in a harmonious dance to ensure a steady supply of energy for cells. Fermentation provides an immediate energy source under oxygen-starved conditions, such as during intense exercise or in the depths of a wound. Cellular respiration, on the other hand, sustains long-term energy production when oxygen is readily available.
Overall, these two pathways demonstrate the remarkable adaptability and efficiency of cellular energy production. Together, they provide cells with the power they need to thrive and carry out their complex life functions.
Similarities Between Fermentation and Cellular Respiration: A Tale of Two Energy Pathways
Amidst the bustling metropolis of a living cell, two metabolic pathways, fermentation and cellular respiration, play pivotal roles in generating the energy that fuels life’s processes. While each pathway treads a distinct path, they share striking similarities that reveal their intricate dance of cooperation.
Common Currency: The Power of ATP
Both fermentation and cellular respiration harness the power of adenosine triphosphate (ATP), the universal currency of cellular energy. ATP acts as a rechargeable battery, providing the instant energy needed for a myriad of cellular activities, from muscle contraction to protein synthesis.
Electron Carriers: NADH and NADPH, the Energy Shuttles
In both pathways, NADH (nicotinamide adenine dinucleotide hydride) and NADPH (nicotinamide adenine dinucleotide phosphate hydride) serve as essential electron carriers. They capture electrons from substrate molecules during the breakdown of glucose, offering their bounty of energy for the synthesis of ATP.
Substrate Level Phosphorylation: A Quick Energy Fix
Fermentation, an anaerobic process, relies on substrate level phosphorylation to generate a limited amount of ATP. This direct approach provides a rapid energy source in the absence of oxygen.
The Oxygen Factor: Anaerobic vs. Aerobic Dance
The key distinction between fermentation and cellular respiration lies in their oxygen dependence. Fermentation, as mentioned earlier, can operate without oxygen, while cellular respiration thrives in its presence. This divide in oxygen usage significantly impacts their metabolic efficiency.
Metabolic Champions: The Efficiency Divide
Cellular respiration reigns supreme in efficiency, boasting a far greater ATP yield than fermentation. This is attributed to the complete oxidation of glucose, a process only possible with ample oxygen.
Interplay of Fermentation and Cellular Respiration: A Symphony of Energy
Far from being rivals, fermentation and cellular respiration work harmoniously to provide a steady stream of energy for the cell. Fermentation serves as a first responder, providing an immediate energy source when oxygen is scarce. Once oxygen becomes available, cellular respiration takes over, offering a sustained and more efficient energy production.
Through their shared mechanisms and complementary roles, fermentation and cellular respiration ensure the uninterrupted flow of energy that powers life’s countless processes.