Understanding Extensive And Intensive Properties For Scientific Analysis
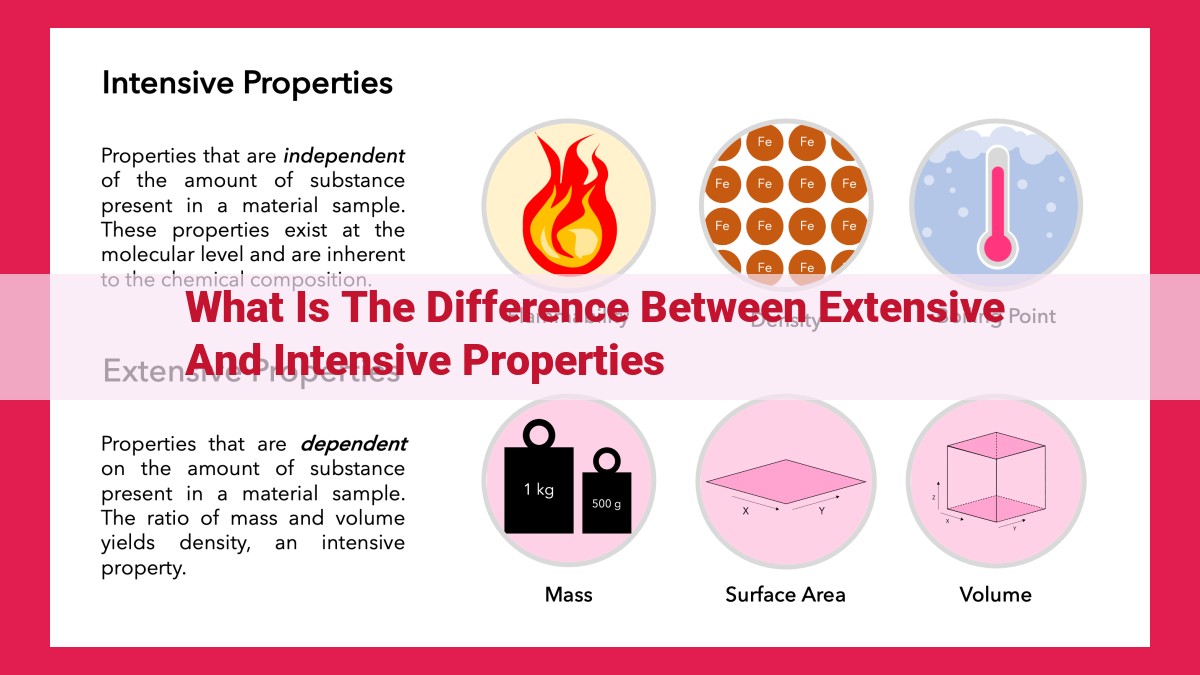
Matter’s properties are categorized into extensive and intensive based on their relation to the amount of matter. Extensive properties, like mass, volume, and energy, depend on the quantity of matter and are additive. Intensive properties, including temperature, pressure, and density, are independent of quantity and concentration. Extensive properties describe the total amount of a substance, while intensive properties describe the characteristics of a specific portion. Understanding these distinctions is crucial for accurate scientific analysis in fields like chemistry and physics.
Understanding the ABCs of Matter: Extensive and Intensive Properties
In the realm of science, we encounter a myriad of properties that describe the characteristics of matter. At the core of these properties lies a fundamental distinction: extensive versus intensive properties. Grasping this difference is crucial for understanding the behavior of matter and its applications in various fields.
Extensive Properties: The Additives
Imagine a vast ocean, its boundless expanse stretching beyond the horizon. Extensive properties, like this colossal body of water, depend on the amount of matter present. Mass, volume, and energy are prime examples. If you add more water to the ocean, its mass, volume, and energy increase proportionately. This additivity is a hallmark of extensive properties.
Intensive Properties: The Constants
In contrast to extensive properties, intensive properties remain constant regardless of the amount of matter. Temperature, pressure, and density are steadfast examples. Consider the temperature of a cup of coffee. Whether you have a sip or a gulp, the temperature stays the same.
Distinguishing the Duo: A Tale of Two
To clarify the distinction, let’s delve into a table that highlights their key differences:
| Feature | Extensive Properties | Intensive Properties |
|---|---|---|
| Dependency on Amount | Dependent | Independent |
| Additivity | Additive | Non-additive |
| Units of Measurement | Depend on amount (e.g., grams, liters) | Independent of amount (e.g., degrees Celsius, atmospheres) |
Practical Applications: Unlocking the Power
These properties play a pivotal role in scientific disciplines. Extensive properties, such as entropy and enthalpy, are essential in thermodynamics. Intensive properties, like pH and solubility, guide chemical reactions and environmental studies. By unraveling the nature of these properties, scientists gain invaluable insights into the intricacies of matter.
Defining Extensive Properties
In the realm of matter’s characteristics, an important distinction lies between extensive and intensive properties. Extensive properties, like mass, volume, and energy, are those that depend on the quantity or amount of substance present. They increase proportionally with the size or amount of the system.
A defining characteristic of extensive properties is their additivity. When you combine two or more systems with the same substance, the extensive property of the combined system is simply the sum of the individual values. For instance, if you have two containers of water, each containing 500 grams of water, the total mass of the combined water is 1000 grams.
The dependence of extensive properties on quantity and concentration is also crucial. As the amount of substance increases, so does the extensive property. Similarly, if the concentration of a substance increases, its extensive properties also increase. For example, the mass of a solution increases when you add more solute to it.
Examples of extensive properties abound in the physical world. Mass, the quantity of matter an object possesses, is an extensive property. Volume, the amount of space occupied by a substance, is another. And energy, the capacity to do work or produce heat, is also an extensive property.
Understanding the nature of extensive properties is essential for a wide range of scientific disciplines, including chemistry, physics, and environmental science. They play a crucial role in calculations involving thermodynamics, such as entropy and enthalpy. They are also important in understanding chemical reactions and phase changes.
Defining Intensive Properties: The Unvarying Characteristics of Matter
In the realm of science, we often encounter two fundamental categories of properties that describe matter: extensive and intensive properties. Intensive properties stand out as distinct and unwavering characteristics that remain unaffected by the amount or quantity of the substance present. Unlike their extensive counterparts, intensive properties exhibit a remarkable independence from the size or concentration of the system.
Non-Additivity: A Defining Trait of Intensive Properties
One of the key features that sets intensive properties apart is their non-additivity. This means that the value of an intensive property for a system as a whole cannot be simply obtained by adding the values of that property for its individual components. For instance, consider the temperature of two separate beakers of water. If you mix these two beakers, the resulting temperature of the combined system will not be the average of the individual temperatures. Instead, it will reach a new equilibrium temperature that is independent of the amount of water in each beaker.
Independence from Quantity and Concentration
Another defining characteristic of intensive properties is their independence from quantity and concentration. This means that the value of an intensive property remains constant regardless of the size or concentration of the system. For example, the density of a substance, which is a measure of its mass per unit volume, remains the same whether you have a small sample or a large quantity of that substance. Similarly, the boiling point of a liquid is an intensive property that is unaffected by the amount of liquid present.
Examples of Intensive Properties
Common examples of intensive properties include:
- Temperature: The measure of the average kinetic energy of particles in a system
- Pressure: The force exerted by a substance per unit area
- Density: The mass per unit volume of a substance
- Solubility: The maximum amount of a substance that can dissolve in a given solvent
- pH: A measure of the acidity or alkalinity of a solution
By understanding the nature and behavior of intensive properties, scientists and researchers can gain valuable insights into the fundamental characteristics of matter and its interactions. These properties play a crucial role in fields such as chemistry, physics, and environmental science, helping us to predict and explain a wide range of phenomena in the world around us.
Key Distinctions between Extensive and Intensive Properties
Understanding the distinction between extensive and intensive properties is vital in comprehending the behavior of matter. While both describe the characteristics of a substance, they differ in how they respond to changes in quantity.
Extensive Properties
- Additive: The value of an extensive property for a system is the sum of the values for its individual components.
- Dependent on Amount: The magnitude of an extensive property increases proportionally with the amount of matter present.
- Units of Measurement: Typically expressed in units that reflect quantity, such as kilograms for mass, liters for volume, and joules for energy.
Examples: mass, volume, length, energy
Intensive Properties
- Non-Additive: The value of an intensive property for a system is independent of the amount of matter present.
- Independent of Quantity: The magnitude of an intensive property remains constant regardless of the size of the system.
- Units of Measurement: Often expressed in units that do not depend on quantity, such as degrees Celsius for temperature, pascals for pressure, and grams per cubic centimeter for density.
Examples: temperature, pressure, pH, solubility
Summarizing Key Differences
| Property | Dependency on Amount | Additivity | Units of Measurement |
|---|---|---|---|
| Extensive | Proportional | Additive | Reflects Quantity |
| Intensive | Independent | Non-Additive | Independent of Quantity |
Applications of Extensive and Intensive Properties: Delving into Thermodynamics and Chemistry
The understanding of extensive and intensive properties extends beyond their theoretical definitions. They find practical applications in diverse scientific disciplines, particularly thermodynamics and chemistry.
Thermodynamics: The Role of Extensive Properties
In the realm of thermodynamics, extensive properties play a crucial role in understanding the behavior of systems undergoing energy transfer. Entropy and enthalpy, two key thermodynamic quantities, are extensive properties. Entropy measures the degree of disorder or randomness within a system, while enthalpy represents the total thermal energy of the system.
The extensive nature of entropy and enthalpy implies that their values scale proportionally with the quantity of matter involved. For instance, if you double the amount of a gas in a container, its entropy and enthalpy will also double. This additivity property makes extensive properties particularly valuable in analyzing large-scale systems.
Chemistry: Exploring Intensive Properties
In the domain of chemistry, intensive properties take center stage. pH, a measure of the acidity or basicity of a solution, is an example of an intensive property. Unlike extensive properties, pH does not depend on the concentration or amount of the solution. Rather, it is an intrinsic property that characterizes the solution’s chemical nature.
Another crucial intensive property in chemistry is solubility. The solubility of a substance refers to its ability to dissolve in a specific solvent. Similar to pH, solubility does not vary with the amount of solute present. It is an inherent characteristic of the solute-solvent pair under specific conditions.
Grasping the nuances of extensive and intensive properties is fundamental to comprehending the behavior of matter in various scientific contexts. From thermodynamics to chemistry, these properties provide invaluable insights into system behavior and facilitate accurate predictions. By harnessing our knowledge of these properties, scientists and engineers can develop innovative solutions to real-world problems and advance our understanding of the world around us.