Unveiling The Significance Of Double Bonds: Enhancing Molecular Strength And Reactivity
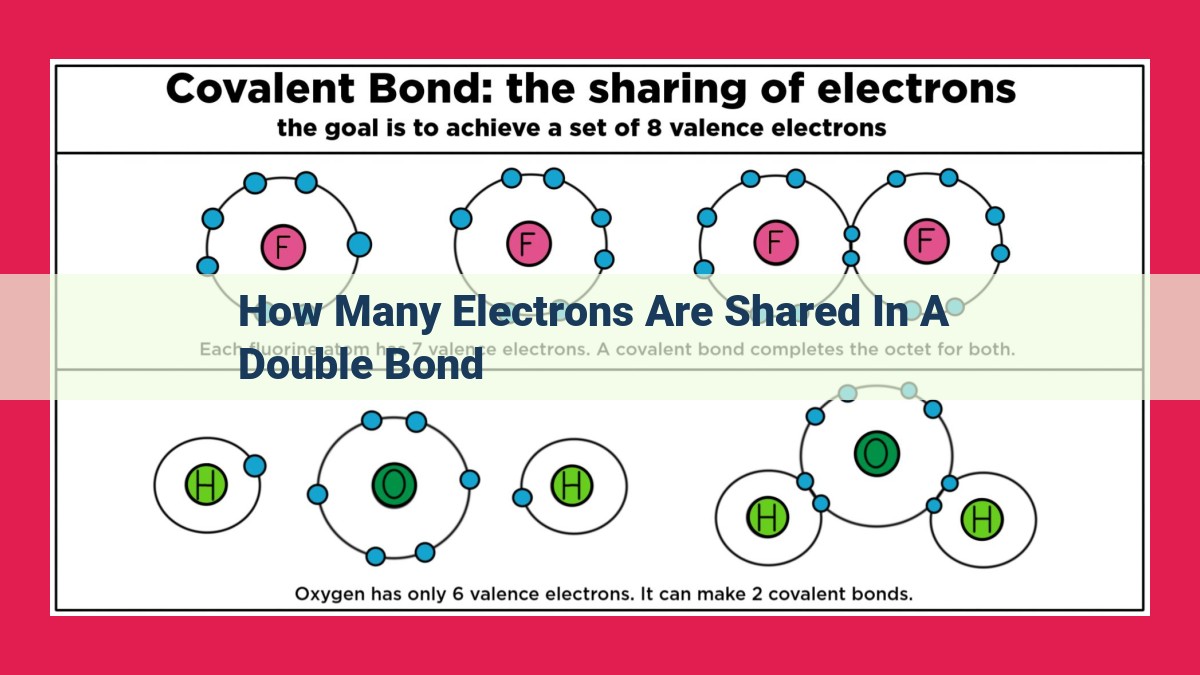
A double bond involves the sharing of four electrons between two atoms, consisting of a strong sigma bond formed from head-to-head overlapping of atomic orbitals and a weaker pi bond resulting from lateral overlap. This shared electron count contributes to the bond strength and stability, leading to a shorter bond length and higher bond order compared to single bonds. Double bonds are crucial in forming various molecules, such as alkenes and carbonyl compounds, and participate in a range of chemical reactions due to the reactivity of their pi bond.
Definition and Significance of Double Bonds:
- Explain what a double bond is and how it differs from single and triple bonds.
- Discuss its importance in chemistry and its role in forming various molecules.
A Guide to Double Bonds: Unveiling Their Chemistry and Significance
Double bonds, prevalent in the chemical world, play a pivotal role in the formation of myriad molecules and dictate their properties. Understanding their essence is paramount for unraveling the intricacies of chemistry.
A double bond is a covalent bond between two atoms that involves two pairs of shared electrons. Unlike single bonds, which share only one pair, double bonds exhibit greater strength and stability. They result from the overlap of atomic orbitals in a specific manner, forming sigma and pi bonds. Sigma bonds, akin to single bonds, arise from the head-on overlap of orbitals, while pi bonds stem from the lateral overlap of orbitals.
Double bonds are ubiquitous in chemistry, featuring in diverse molecules such as alkenes, ketones, and carboxylic acids. Their presence influences the reactivity of these molecules, especially due to the presence of the reactive pi bond. This pi bond renders double bonds susceptible to a range of reactions, including addition, substitution, and cycloadditions, which are crucial in organic synthesis.
In addition to their reactivity, double bonds have significant applications in chemistry. They serve as building blocks for polymers and plastics, imparting unique properties to these materials. Furthermore, double bonds find use in the synthesis of pharmaceuticals and other chemicals. Their versatility and importance make them indispensable tools in the hands of chemists.
By comprehending the nature and significance of double bonds, we gain a deeper appreciation for the intricate world of chemistry. These bonds, like hidden treasures, reveal the secrets of molecular structure, reactivity, and applications, driving the advancements of science and technology.
The Intriguing World of Double Bonds: A Deep Dive into Their Formation
In the vast realm of chemistry, bonds play a pivotal role in shaping the molecules that make up our world. Among these bonds, the double bond stands out as a captivating entity that holds profound significance in the dance of atoms. Let’s embark on a captivating journey to unravel the intricate tapestry of double bond formation.
To comprehend double bonds, it’s paramount to grasp their fundamental nature. A double bond arises when two atoms share four electrons, resulting in an extraordinary sharing arrangement beyond the typical two electrons in single bonds. This intimate sharing of electrons establishes a stronger bond than a single bond, enhancing the stability of molecules and opening up avenues for remarkable chemical transformations.
The formation of a double bond is a delicate dance of atomic orbitals. When atoms approach each other with the intention of forming a double bond, their atomic orbitals undergo a hybridization process. For instance, in the case of a carbon-carbon double bond, each carbon atom undergoes sp² hybridization. This hybridization gives rise to three equivalent sp² hybrid orbitals that lie in a single plane, forming a triangular arrangement around the carbon nucleus.
In the subsequent step, the head-on overlap of two sp² hybrid orbitals from each carbon atom results in the formation of a strong sigma (σ) bond. This σ bond represents the foundation of the double bond, providing the primary route for electron sharing.
Simultaneously, the unhybridized p orbitals on each carbon atom overlap laterally, forming a unique bond known as a pi (π) bond. The π bond lies above and below the plane of the σ bond, completing the double bond’s characteristic structure.
Double bonds are distinguished by their shorter bond length and higher bond order compared to single bonds. The shorter bond length reflects the enhanced electron sharing, while the higher bond order signifies the presence of two bonds (a σ bond and a π bond) between the atoms.
The captivating dance of atomic orbitals in double bond formation paves the way for the remarkable properties and reactivity that double bonds possess. Delve into subsequent sections to unravel these fascinating aspects and discover the boundless applications of double bonds in the enchanting world of chemistry.
Properties of Double Bonds: Strength, Stability, and Geometry
In the realm of chemistry, double bonds play a pivotal role in forming countless molecules and underpinning their chemical behavior. Their unique properties, markedly different from single bonds, give them remarkable strength, stability, and a characteristically shorter bond length.
Enhanced Strength and Stability
Double bonds possess greater strength than single bonds due to the presence of two shared electron pairs between the bonded atoms. This additional electron density creates a stronger electrostatic attraction, making double bonds more resistant to breaking.
Moreover, double bonds exhibit increased stability because they are less prone to rotation than single bonds. The pi bond, which is responsible for the double bond character, restricts rotation around the bond axis. This restricted rotation provides additional stability to the double bond.
Shorter Bond Length and Higher Bond Order
Double bonds are shorter than single bonds because the shared electrons are held closer to the nuclei. This reduced bond length reflects the stronger attraction between the bonded atoms.
Additionally, double bonds have a higher bond order than single bonds. Bond order represents the number of electron pairs shared between atoms. Single bonds have a bond order of 1, while double bonds have a bond order of 2. The higher bond order indicates the greater strength and stability of double bonds.
In summary, double bonds exhibit enhanced strength and stability compared to single bonds. Their shorter bond length and higher bond order further underscore their unique properties, which are essential for understanding their reactivity and the formation of various molecules.
Types and Examples of Double Bonds: A Journey through the Realm of Chemical Connectivity
In the realm of chemistry, double bonds hold a pivotal position, connecting atoms with a unique dual character. Unlike their single-bonded counterparts, these strong and stable chemical alliances open up a world of possibilities. Let’s embark on an adventure to explore the diverse types and captivating examples of double bonds.
Carbon-Carbon (C=C) Double Bonds: The Backbone of Organic Chemistry
The most prevalent type of double bond is the carbon-carbon (C=C) double bond. This connection forms the backbone of countless organic molecules, playing a key role in the intricate structures of alkenes, the building blocks of plastics. Their reactivity and versatility make them crucial in various industrial processes and the synthesis of complex compounds.
Carbon-Heteroatom (C=O) Double Bonds: Expanding the Chemical Horizon
Beyond carbon-carbon double bonds, the chemical landscape expands to include carbon-heteroatom (C=O) double bonds. These alliances between carbon and elements like oxygen, nitrogen, and sulfur create a rich tapestry of functional groups. Aldehydes, ketones, and carboxylic acids proudly display these double bonds, bestowing upon them distinct chemical properties and the ability to participate in a wide range of reactions.
Examples that Paint a Picture: Molecules Adorned with Double Bonds
To illustrate the diversity of double bonds, consider the following molecular examples:
- Ethylene (ethene): A simple yet fundamental alkene, ethylene boasts a carbon-carbon double bond that forms the foundation of countless polymers and plastics.
- Acetone: A ubiquitous ketone, acetone exhibits a carbon-oxygen double bond that grants it the ability to dissolve various substances and serve as a solvent.
- Acetic acid: A carboxylic acid essential for life, acetic acid owes its acidic properties to the carbon-oxygen double bond within its functional group.
The world of chemistry resonates with the harmonious interplay of double bonds, each type contributing its unique voice to the symphony of molecular structures. Their strength, stability, and reactivity make them indispensable partners in countless chemical processes and everyday materials. Understanding these versatile connections unlocks the gateway to a deeper appreciation of the intricate dance of atoms in the chemical realm.
The Enchanting Reactivity of Double Bonds: A Chemical Tale of Wonders
In the realm of chemistry, double bonds stand out as alluring and enigmatic entities, possessing a remarkable reactivity that sets them apart from their single- and triple-bonded brethren. This enhanced reactivity stems from the presence of a reactive pi bond, a bond that opens up a world of possibilities for chemical reactions.
Imagine two dancers, their arms intertwining to form an intricate double bond. The pi bond, like the supple bodies of our dancers, is a sideways overlap of atomic orbitals, creating a cloud of electrons that hovers above and below the molecular plane. This electron-rich environment makes double bonds highly susceptible to a variety of chemical reactions.
Addition Reactions: One of the most common reactions involving double bonds is addition. Picture a thirsty plant eagerly absorbing water from the soil. In addition reactions, two atoms or molecules eagerly attach themselves to the double bond, forming a new, saturated compound. These reactions are often used to create new molecules with desired properties.
Substitution Reactions: In substitution reactions, a hungry atom or molecule swaps places with one of the atoms in the double bond. Think of a mischievous child replacing their sibling with a plush toy. Substitution reactions can be used to modify existing molecules and introduce new functional groups.
Cycloadditions: These reactions are the chemical equivalent of a perfect puzzle fit. Two molecules with double bonds come together like puzzle pieces, forming a ring-shaped molecule. Cycloadditions are essential for the synthesis of many complex compounds, including pharmaceuticals and natural products.
The reactivity of double bonds is not just a quirk of their nature; it’s a blessing to chemists worldwide. Double bonds are the building blocks of countless organic molecules, including plastics, pharmaceuticals, and fragrances. Their ability to undergo a diverse range of reactions makes them indispensable tools for the creation of new materials and the advancement of scientific research.
Applications of Double Bonds:
- Highlight the various uses of double bonds in chemistry, including their importance in the synthesis of organic molecules.
- Discuss their applications in polymers, plastics, and pharmaceutical compounds.
Applications of Double Bonds: A Tale of Versatility
In the realm of chemistry, double bonds reign as masters of versatility, showcasing their significance in countless ways. Their applications extend far beyond the confines of theoretical concepts, reaching into the practical world of everyday use.
Synthesis of Organic Molecules: A Symphony of Creation
Double bonds serve as the building blocks of countless organic molecules, molecules that form the backbone of life itself. Chemists harness the power of double bonds to meticulously craft these molecules, carefully orchestrating their chemical dance to create everything from pharmaceuticals to fragrances.
Polymers: The Backbone of Modern Materials
Double bonds are the secret ingredient that gives polymers their remarkable strength and flexibility. These materials, ubiquitous in our lives, owe their very existence to the presence of these crucial double bonds. From lightweight plastics to durable construction materials, polymers find their place in countless industries.
Pharmaceutical Compounds: A Cure Unraveled
In the field of medicine, double bonds play a pivotal role in the development of pharmaceutical compounds. By cleverly exploiting the reactivity of double bonds, scientists can design and synthesize drugs that target specific ailments, offering hope and healing to those in need.
In essence, double bonds are the versatile workhorses of chemistry, enabling the creation of myriad compounds that enhance our lives in countless ways. They are the unsung heroes of modern society, their contributions often unnoticed, but always present, shaping the very fabric of our world.