Understanding The Dna Molecule: Decoding Structure And Function
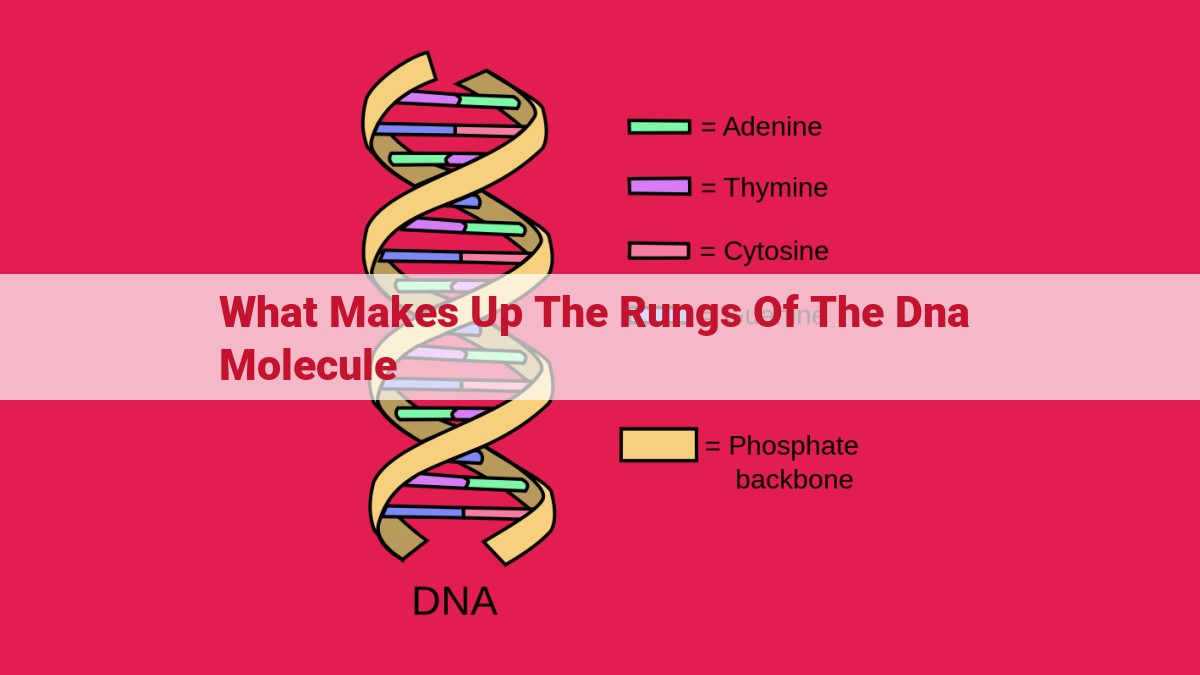
The DNA molecule comprises deoxyribose sugar, phosphate groups, and nitrogenous bases. Deoxyribose sugar forms the backbone of the double helix, providing structural support. Phosphate groups, attached to deoxyribose, maintain the double helix structure through electrostatic repulsion. Nitrogenous bases (adenine, thymine, cytosine, and guanine) form the “rungs” of the DNA ladder, pairing with each other (A with T, C with G) through hydrogen bonds, providing stability and enabling the storage and transmission of genetic information.
The Backbone of the DNA Double Helix: Deoxyribose Sugar
Picture the blueprint of life, a molecule so intricate and awe-inspiring that it holds the secrets to our very existence: DNA. Unraveling its structure feels akin to embarking on an extraordinary journey, and today, we’ll delve into the backbone that forms the foundation of this remarkable molecule – deoxyribose sugar.
Think of deoxyribose sugar as the thread that weaves together the strands of DNA. It’s a sugar molecule that, along with phosphate groups, forms the scaffold for the double helix. Each deoxyribose sugar molecule is attached to a phosphate group, creating a repeating pattern that resembles a twisted ladder.
Now, imagine these sugar-phosphate units as the beads on a necklace. Each bead is a nucleotide, the basic building block of DNA. Nucleotides consist of three components: a deoxyribose sugar, a phosphate group, and a nitrogenous base. It’s the nitrogenous bases that give DNA its ability to store genetic information, but we’ll delve into that fascinating tale later.
For now, let’s focus on the role of deoxyribose sugar in providing the backbone’s stability. The deoxyribose sugar molecule has a unique structure with five carbon atoms, making it more rigid than other sugars. This rigidity is crucial for maintaining the double helix shape and preventing DNA from collapsing in on itself.
The Supporting Structure: Phosphate Group
In the intricate architecture of DNA, the phosphate group plays a vital role as the connective tissue that holds the double helix together. Residing within the backbone of the DNA molecule, this tiny chemical plays a mighty part in providing structural stability and preserving the genetic information encoded within its sequence.
Nucleotides, the building blocks of DNA, consist of three components: deoxyribose sugar, phosphate group, and a nitrogenous base. These nucleotides are arranged in a linear fashion, with the phosphate group of one nucleotide linking to the deoxyribose sugar of the next. This interlinking chain forms the backbone of the DNA helix.
The phosphate group possesses a negative charge, which creates an electrostatic repulsion between adjacent strands of the DNA double helix. This repulsion serves as a force that keeps the two strands apart, maintaining the double helix structure.
Without the phosphate group, the DNA molecule would collapse into a single strand, compromising its stability and hindering its ability to store and transmit genetic information. The electrostatic repulsion generated by the phosphate group ensures that the double helix remains intact, safeguarding the integrity of the genetic code.
The Rungs of the Double Helix: Nitrogenous Bases
Imagine the DNA molecule as an intricate ladder, with its backbone formed by interlocking sugar molecules and phosphate groups. The rungs of this ladder are composed of nitrogenous bases, the building blocks of genetic information. These bases come in four distinct varieties: adenine (A), thymine (T), cytosine (C), and guanine (G).
The magic of DNA lies in its complementary base pairing. Adenine always pairs with thymine, forming a strong bond through two hydrogen bonds. Likewise, cytosine exclusively pairs with guanine, forming a triple-hydrogen-bond bond. This precise pairing ensures that the two strands of DNA complement each other perfectly, creating a stable double helix structure.
Furthermore, the nitrogenous bases are hydrophobic, meaning they repel water. This characteristic contributes to the overall stability of the DNA molecule, as the hydrophobic bases are tucked away on the inside, protected from water.
Through base pairing, hydrogen bonds form between the nitrogenous bases on opposite strands, holding the DNA double helix together like a zipper. These bonds provide the energy required to maintain the DNA’s structure and allow it to unwind and replicate during cell division.
In summary, the nitrogenous bases serve as the rungs of the DNA ladder, providing the genetic information and ensuring the stability of this vital molecule. Through their complementary base pairing, they create a double helix structure that allows for the replication and transmission of genetic information generation after generation.