Understanding Covalent Compounds: Sharing Electrons For Molecular Formation
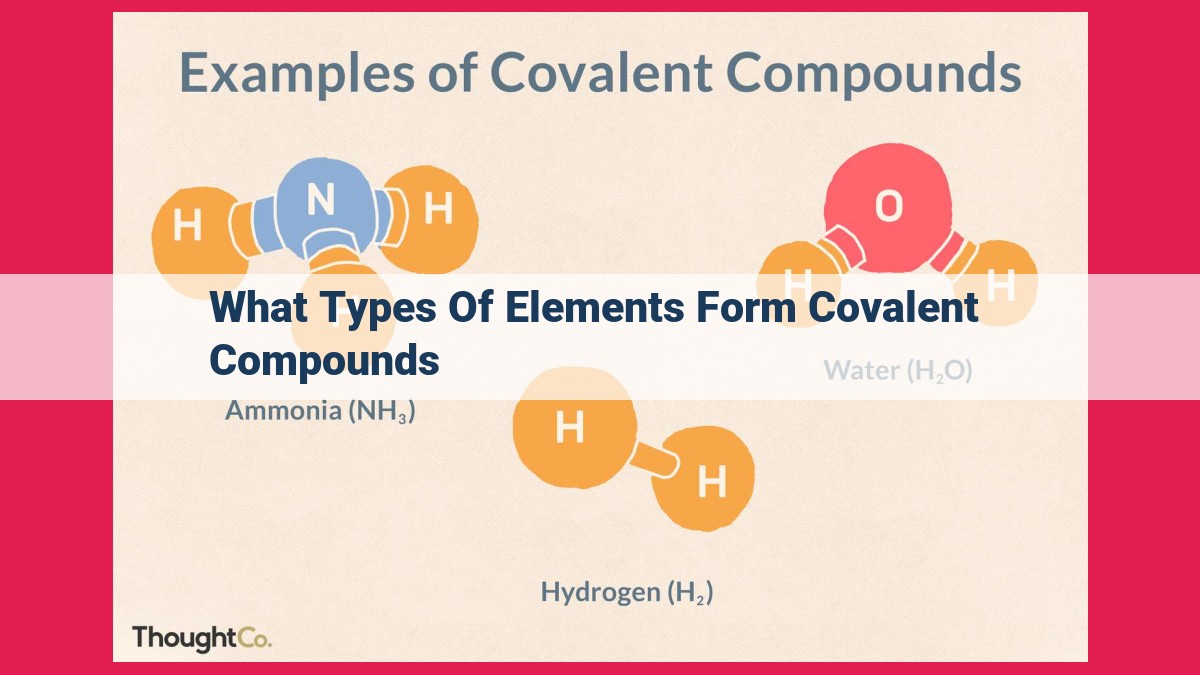
Covalent compounds arise from the sharing of electrons between atoms, primarily nonmetals. Nonmetals, located on the right side of the periodic table, possess high electronegativity, a measure of their attraction for electrons. When nonmetals interact, their similar electronegativities result in covalent bonds where electrons are shared equally, forming nonpolar covalent bonds. However, if the electronegativities differ significantly, the electrons are unequally distributed, creating polar covalent bonds with partial charges. The combination of nonmetal atoms and electron sharing leads to molecular compounds characterized by low melting and boiling points due to the weak intermolecular forces holding them together.
Covalent Compounds: The Enigmatic Electron Sharers
Covalent compounds, the enigmatic players in the chemical realm, are formed when atoms join hands, not through the exchange of electrons, but through an intricate dance of electron sharing. This unique bond, captivating in its essence, forms the foundation of a plethora of molecules that grace our world.
These covalent bonds are the result of a delightful dance between nonmetal atoms, elements that reside on the periodic table’s right-hand side. These atoms possess a remarkable trait: high electronegativity, a measure of their insatiable appetite for electrons. This hunger drives them to seek out partners with whom they can share their electron wealth.
When nonmetals encounter each other, their electronegativities engage in a delicate waltz. If their electronegativities are similar, they strike an equal partnership, forming a nonpolar covalent bond characterized by an equitable distribution of electrons. However, when their electronegativities differ, a more unequal alliance emerges, giving birth to a polar covalent bond marked by an uneven distribution of electrons. These polar covalent bonds introduce a fascinating dimension, creating partial charges within the molecule, bestowing it with a dipole moment.
Covalent compounds, united by these electron-sharing bonds, manifest as molecular compounds, entities composed exclusively of nonmetal atoms. These compounds often flaunt low melting points and boiling points, attributes that stem from the relatively weak intermolecular forces that hold them together.
In the symphony of chemical reactions, covalent compounds play a pivotal role. Their unique bonding characteristics make them essential components of countless substances that enrich our lives, from the medicines that heal us to the plastics that shape our world.
So, let us celebrate the power of electron sharing, the driving force behind covalent compounds, the enigmatic architects of countless molecules that grace our planet.
Nonmetals: The Cornerstones of Covalent Bonding
In the realm of chemistry, the formation of covalent bonds is a fundamental concept that underlies the existence of numerous substances around us. These remarkable compounds are characterized by the intimate sharing of electrons between atoms, giving rise to their unique properties. At the heart of this covalent bonding phenomenon lie nonmetals, the unsung heroes of the chemical world.
Nonmetals: Setting the Stage
Nonmetals occupy the right-hand side of the periodic table, extending from the top corner (hydrogen) to the bottom right (radon). These elements are defined by their lack of metallic characteristics, such as ductility, malleability, and luster. Instead, they exhibit properties that set them apart from their metallic counterparts:
-
High Electronegativity: Nonmetals have a strong affinity for electrons. Their atoms readily attract electrons toward themselves, a crucial factor in the formation of covalent bonds.
-
Location on the Periodic Table: Nonmetals are typically found in groups 14 to 17. Moving from left to right across a period, the electronegativity of nonmetals increases, making them increasingly likely to share electrons and form covalent bonds.
Electronegativity: The Key to Chemical Bond Formation
Electronegativity is a measure of an atom’s ability to attract electrons towards itself. This force is crucial in determining the type of chemical bonds formed between atoms.
When two atoms have similar electronegativities, they share electrons equally, forming a nonpolar covalent bond. This bond is stable because the electrons are evenly distributed between the atoms. An example of a nonpolar covalent bond is the H-H bond in hydrogen gas.
However, when two atoms have different electronegativities, the atom with the higher electronegativity will attract electrons more strongly. This creates an unequal distribution of electrons, resulting in a polar covalent bond. In a polar covalent bond, one atom has a partial negative charge and the other atom has a partial positive charge. The difference in electronegativity determines the magnitude of the partial charges.
The difference in electronegativity also dictates the type of bond formed. A small difference in electronegativity leads to a slightly polar covalent bond, while a large difference leads to a highly polar covalent bond. In some cases, the difference in electronegativity can be so great that it results in the formation of an ionic bond, where one atom completely transfers an electron to the other.
Understanding electronegativity is essential for comprehending the formation and properties of chemical bonds. It helps us predict the type of bond formed between two atoms, as well as the polarity of that bond. This knowledge is fundamental for understanding the behavior and interactions of chemical substances.
Covalent Bond: The Essence of Electron Sharing
In the realm of chemistry, the dance of electrons holds a captivating secret – the covalent bond. This unique bond is the heart of molecular compounds, the building blocks of innumerable substances that shape our world. Let’s embark on an enchanting journey to unravel the mysteries of covalent bonds, exploring the magic of electron sharing.
Covalent bonds are formed when nonmetal atoms, eager to gain stability, come together. These atoms, like kindred spirits, yearn to complete their outermost electron shells, the outermost layer of electrons surrounding their nucleus. They achieve this harmony by sharing electrons, forming a shared electron pair that dances around both nuclei, creating a covalent bond.
The strength of a covalent bond lies in the electronegativity of the participating atoms. Electronegativity, a measure of an atom’s pull on shared electrons, determines the electron distribution within the bond. If the electronegativity of the atoms is similar, the electrons are shared equally, forming a nonpolar covalent bond. The shared electrons reside happily in the space between the nuclei, creating a harmonious balance.
When the electronegativity of the atoms differs significantly, the electron distribution becomes uneven, leading to a polar covalent bond. The more electronegative atom draws the shared electrons closer to its nucleus, creating a partial negative charge. The less electronegative atom, in turn, acquires a partial positive charge. This polarity imbues the molecule with a distinctive electrical asymmetry.
In essence, covalent bonds are the glue that holds molecular compounds together. They bestow these compounds with unique properties, such as low melting and boiling points, making them vital components in our daily lives. From the plastics that shape our utensils to the pharmaceuticals that heal our ailments, covalent compounds touch every aspect of our existence.
Unveiling the secrets of covalent bonds opens a window to the intricate world of chemistry, revealing the intricate dance of electrons that shape our surroundings. By understanding these fundamental bonds, we gain a deeper appreciation for the wonders of the natural world and the remarkable power of science.
Polar Covalent Bonds: Unequal Distribution of Electrons
In the realm of chemistry, understanding covalent bonds is crucial. These bonds arise when nonmetal atoms share their electrons in a harmonious dance. However, not all covalent bonds are created equal. Some exhibit a peculiar characteristic known as polarity, introducing an exciting twist to the story.
Electronegativity: The Key to Polarity
Just as people have different personalities, atoms possess a distinct property called electronegativity. This property measures an atom’s eagerness to attract shared electrons towards itself. When atoms with different electronegativities join forces in a covalent bond, an uneven distribution of electrons occurs.
Partial Charges: The Result of Unequal Attraction
Imagine a tug-of-war between two atoms, each pulling on the shared electrons with varying strength. The atom with higher electronegativity will exert a stronger pull, attracting the electron cloud closer to itself. This creates an imbalance, resulting in partial charges. The more electronegative atom gains a partial negative charge, while the less electronegative atom acquires a partial positive charge.
Polar Covalent Bonds: A Symphony of Imbalance
Covalent bonds exhibiting this unequal distribution of electrons are known as polar covalent bonds. These bonds form when the electronegativity difference between the bonded atoms is significant but not extreme enough to cause complete electron transfer, resulting in an ionic bond.
Hydrogen Chloride: A Classic Example
Let’s delve into a real-world example. Hydrogen chloride (HCl) is a molecule formed by the covalent bond between a hydrogen atom and a chlorine atom. Chlorine, with its high electronegativity, attracts the shared electrons more strongly, creating a partial negative charge on the chlorine atom and a partial positive charge on the hydrogen atom. This asymmetry distinguishes HCl as a polar covalent compound.
Nonpolar Covalent Bonds: The Essence of Equal Electron Sharing
In the realm of chemistry, the formation of chemical bonds holds paramount importance. One such bond, the nonpolar covalent bond, stands apart as a testament to the power of electron sharing. Unlike other types of bonds, nonpolar covalent bonds are characterized by a harmonious distribution of electrons between atoms.
Nonpolar covalent bonds arise when atoms possess equal electronegativities. Electronegativity measures an atom’s ability to attract electrons toward itself. When atoms have the same electronegativity, they exhibit similar affinities for electrons, resulting in a balanced sharing of these negatively charged particles.
This equal sharing of electrons leads to the formation of a nonpolar covalent bond, which represents a stable and harmonious connection between atoms. The electrons are not distorted towards any one atom, creating a neutral charge distribution.
A classic example of a nonpolar covalent bond is the bond between two identical atoms, such as two hydrogen atoms (H-H) or two chlorine atoms (Cl-Cl). In these cases, the electronegativities of the atoms are identical, leading to a perfect balance of electron sharing.
Nonpolar covalent bonds are the backbone of many elemental molecules, such as hydrogen gas (H2) and chlorine gas (Cl2). These molecules are composed solely of nonpolar covalent bonds, contributing to their low melting and boiling points. As the electron distribution is even throughout the molecules, there are no strong intermolecular forces to hold them together.
In summary, nonpolar covalent bonds represent the epitome of electron sharing, characterized by an equal distribution of electrons between atoms with the same electronegativity. The resulting bonds are harmonious and stable, giving rise to elemental molecules with unique properties.
Molecular Compound: A Symphony of Nonmetal Atoms:
- Define molecular compounds and their composition.
- Discuss the low melting and boiling points of molecular compounds.
Molecular Compounds: A Symphony of Nonmetal Atoms
In the realm of chemistry, where atoms dance and form intricate bonds, molecular compounds emerge as enchanting symphonies of nonmetal atoms. These compounds, composed solely of nonmetals from opposite sides of the periodic table, possess unique characteristics that set them apart from their ionic and metallic counterparts.
One defining feature of molecular compounds is their nonpolar nature. This arises from the equal sharing of electrons between the participating atoms. Unlike ionic bonds, where electrons are fully transferred, covalent bonds in molecular compounds involve a harmonious dance of electrons, creating a balanced distribution of charge.
This equal electron sharing results in a neutral molecule with no net charge. This neutrality extends to the individual atoms within the molecule, as the electrons are shared equally, preventing the formation of charged ions. As a consequence, molecular compounds generally have low melting and boiling points.
The low melting and boiling points of molecular compounds stem from the weak intermolecular forces that exist between the molecules. These forces, such as van der Waals forces and dipole-dipole interactions, are much weaker than the strong electrostatic forces that bind ions together in ionic compounds.
The weak intermolecular forces allow molecular compounds to exist as gases, liquids, or solids at relatively low temperatures and pressures. This versatility makes molecular compounds essential for a wide range of applications, from the fuels that power our vehicles to the plastics that shape our modern world.