Understanding The Conjugate Acid Of Oh: H3O+ And Its Acid-Base Properties
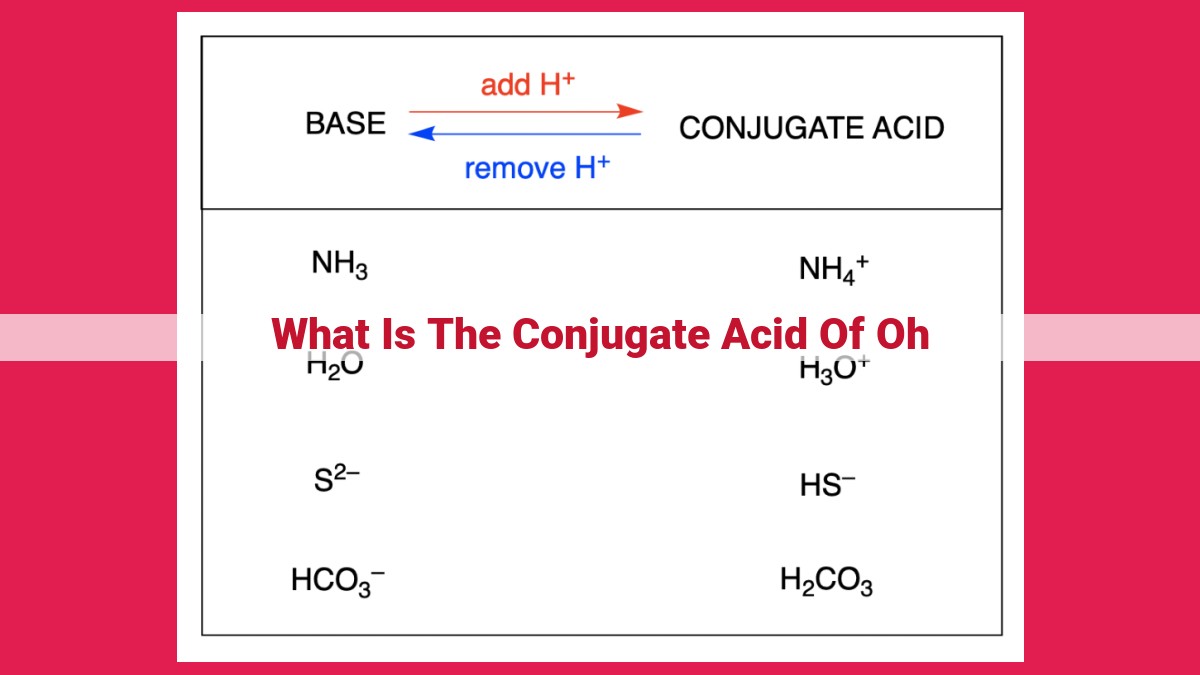
The conjugate acid of OH is H3O+, or the hydronium ion. A conjugate acid is a species formed when a base accepts a proton. OH is a strong base, and when it accepts a proton, it forms H3O+, which is a weak acid. H3O+ is a positively charged ion with a tetrahedral structure. It is a strong acid and readily donates protons to other molecules. H3O+ plays a crucial role in acid-base reactions, including neutralization reactions with bases and proton transfer reactions.
The Intriguing World of Conjugate Acids: Unraveling the Mystery
In the realm of chemistry, acids and bases engage in a captivating dance, exchanging protons like partners on a waltz floor. This dynamic relationship gives rise to a concept known as the conjugate acid. But what exactly is a conjugate acid, and how does it relate to its parent base?
Imagine a base as a substance with a proclivity for donating protons, like a generous host offering its guests a drink. When a base donates a proton, it transforms into its conjugate acid, a substance that is eager to reacquire that lost proton. This proton exchange is like a playful game of tag, where the base releases a proton and the conjugate acid chases after it, keen to reclaim its lost charge.
The strength of an acid or base is determined by its Ka, a value that measures its tendency to donate or accept protons. The stronger the acid, the more readily it donates protons, while the weaker the acid, the less inclined it is to part with its protons. A strong base, on the other hand, readily accepts protons, while a weak base has a lesser affinity for protons.
The relationship between an acid and its conjugate base is a symbiotic one, where the strength of one determines the strength of the other. A strong acid gives rise to a weak conjugate base, while a strong base creates a weak conjugate acid. It’s like a see-saw: as one side goes up, the other goes down. This delicate balance ensures that the chemical equilibrium in a solution is maintained.
The Conjugate Acid of OH: Unraveling the Mystery of the Hydronium Ion
In the realm of chemistry, understanding the concept of conjugate acid-base pairs is crucial. Among these pairs, the conjugate acid of the hydroxide ion (OH-) holds a special significance, unveiling the nature of the enigmatic hydronium ion.
The Hydroxide Ion and Its Conjugate Acid
The hydroxide ion, with its negative charge and lone pair of electrons, is a veritable base. Its conjugate acid, on the other hand, is the positively charged hydronium ion (H3O+). This relationship stems from the protonation of the hydroxide ion, wherein it accepts a proton (H+).
Unveiling the Hydronium Ion: Structure and Properties
The hydronium ion, often represented as H3O+, is a fascinating molecule characterized by its unique structure. It comprises a central oxygen atom bonded to three hydrogen atoms, forming a trigonal pyramidal geometry. This structure grants the ion a permanent dipole, contributing to its polar nature.
As an acid, the hydronium ion ranks among the strongest, boasting a pKa value of -1.74. Its strength stems from its exceptional ability to donate its protons, initiating acid-base reactions with aplomb.
Exploring the Properties of the Hydronium Ion
- Proton Donor: The hydronium ion’s inherent acidity arises from its proclivity to donate protons. It readily engages in proton transfer reactions, relinquishing its H+ to other species.
- Role in Acidic Solutions: In acidic solutions where H3O+ predominates, it dictates the solution’s acidity level. Its concentration directly corresponds to the pH of the solution, serving as a measure of the solution’s acidity.
The Reactivity of the Hydronium Ion
- Neutralization Reactions: The hydronium ion plays a pivotal role in neutralization reactions, where it reacts with hydroxide ions (OH-) to form water molecules. This reaction is the cornerstone of acid-base titrations, enabling the precise determination of acid concentrations.
- Proton Transfer Reactions: H3O+ actively participates in proton transfer reactions, donating its protons to other molecules that act as bases. These reactions are ubiquitous in chemistry, driving a myriad of processes.
Understanding the Importance of the Hydronium Ion in Chemistry
The hydronium ion, as the conjugate acid of OH-, stands as a cornerstone of acid-base chemistry. Its unique structure, strong acidity, and ability to engage in proton transfer reactions make it an indispensable player in numerous chemical processes.
From the determination of solution acidity to the initiation of chemical reactions, the hydronium ion exerts a profound influence on the chemical landscape. By unraveling the mysteries surrounding this intriguing molecule, we unlock a deeper comprehension of the fundamental principles that govern chemical interactions.
The Hydronium Ion: The Powerhouse Behind Acid-Base Reactions
When it comes to acid-base chemistry, one of the most important concepts to understand is the role of the hydronium ion, H3O+. This ion, often referred to as the protagonist of acid-base chemistry, plays a crucial role in various acid-base reactions and is responsible for the acidic properties of aqueous solutions.
Unveiling the Hydronium Ion
Imagine a tiny, positively charged molecule, composed of three hydrogen atoms and one oxygen atom, bound together in a triangular shape. This is the essence of the hydronium ion, H3O+. The hydrogen atoms form covalent bonds with the oxygen atom, creating a tetrahedral geometry. However, due to the presence of an extra hydrogen atom, the hydronium ion bears a positive charge, giving it a unique character.
A Force to Be Reckoned With – The Strength of H3O+
The hydronium ion is a force to be reckoned with when it comes to acidity. Its strength as an acid is measured by its acidity constant, Ka, which is a measure of its ability to donate protons (H+ ions). The lower the Ka value, the stronger the acid. And guess what? The hydronium ion boasts an incredibly low Ka, making it an extremely strong acid.
This exceptional acidity is due to the stability of the conjugate base, water (H2O). When H3O+ donates a proton, it forms H2O, a weak base. The equilibrium between H3O+ and H2O lies heavily towards H3O+, resulting in a high concentration of H3O+ ions in aqueous solutions and contributing to their acidic nature.
Properties of Hydronium Ion (H3O+): A Strong Acid and Proton Donor
The hydronium ion, designated as H3O+, is the conjugate acid of hydroxide ion (OH-), making it integral to acid-base chemistry. Its distinct characteristics contribute to its significance as a strong acid and proton donor.
Acidic Nature: H3O+ possesses a high acidity due to its tendency to donate protons. This property enables it to react with bases, resulting in neutralization reactions that produce water and salt. The strength of its acidity is attributed to the stable oxonium ion structure it forms upon proton donation.
Proton Donation: As a proton donor, H3O+ plays a crucial role in proton transfer reactions. In these reactions, H3O+ donates a proton to a base, leading to the formation of a conjugate acid and water. This process is essential in acid-base titrations and other chemical reactions involving proton transfer.
Additional Properties:
- Solubility: H3O+ is highly soluble in water, facilitating its significant presence in aqueous solutions.
- Reactivity: H3O+ reacts with a wide range of compounds, including metals, oxides, and carbonates.
- Stability: The oxonium ion structure of H3O+ contributes to its stability, allowing it to exist in aqueous solutions without decomposing.
In conclusion, hydronium ion (H3O+) is a strong acid and proton donor with unique properties that make it essential in acid-base reactions. Its acidity, proton donation ability, and other characteristics underline its importance in various chemical processes.
Reactions of the Hydronium Ion (H(^+))
In the realm of chemistry, the hydronium ion (H(^+)) plays a pivotal role in countless reactions. This extraordinary ion, the conjugate acid of the hydroxide ion (OH(^-)), exhibits a remarkable array of chemical behaviors, rendering it an indispensable player in acid-base equilibria.
One of the most fundamental reactions of H(^+) is its participation in neutralization reactions. When H(^+) encounters a base, such as sodium hydroxide (NaOH), it undergoes a proton transfer reaction, resulting in the formation of water and a salt. For instance:
H\(^+\) + NaOH → HOH + Na\(^+\)
This reaction lies at the heart of acid-base titrations, a technique employed to determine the concentration of an unknown acid or base.
Beyond neutralization reactions, H(^+) also engages in proton transfer reactions, serving as a proton donor to other chemical species. This ability underlies its strong acidic nature. In aqueous solutions, H(^+) readily donates a proton to water molecules, forming more hydronium ions:
H\(^+\) + HOH → H\(^+\) + OH\(^-\)
This process contributes to the autoionization of water, a phenomenon responsible for the presence of both H(^+) and OH(^-) ions in pure water.
Moreover, H(^+) can transfer protons to organic molecules, facilitating a wide range of chemical transformations. For example, it can protonate amines to form ammonium ions and promote the hydrolysis of esters and amides.
In summary, the hydronium ion (H(^+)) is a highly reactive species that plays a crucial role in various chemical reactions. Its ability to participate in neutralization and proton transfer reactions makes it an essential player in acid-base chemistry and numerous other chemical processes.