Understanding Buffers: Maintaining Stable Ph In Chemical Systems
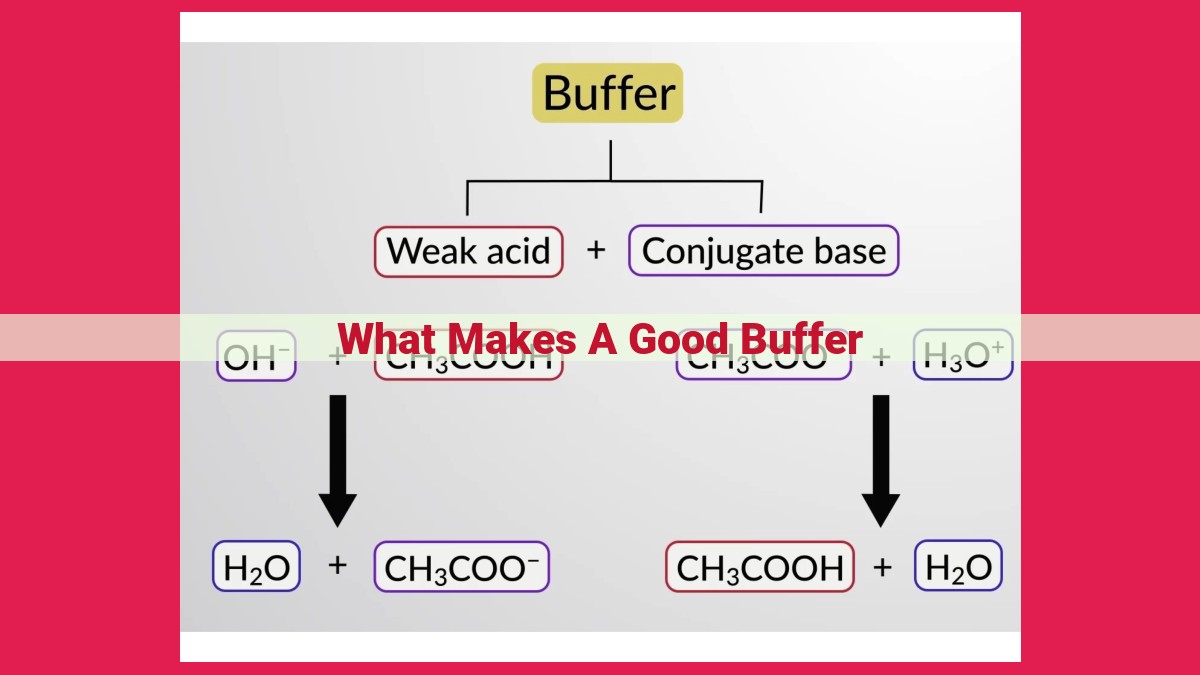
A good buffer effectively resists pH changes, maintaining a stable pH within a specific range. It consists of a weak acid and its conjugate base, with balanced concentrations that determine the buffer capacity. The pH of the buffer is set by the dissociation constant of the weak acid, with a buffer range usually within 1 pH unit below and above the pKa. Higher concentrations of the buffer components enhance buffering capacity, allowing it to withstand larger pH variations. A wider pH range indicates the buffer’s ability to maintain stable pH over a larger range of added acids or bases.
Understanding Buffers: The Guardians of pH Stability
In the realm of chemistry, pH plays a crucial role in determining the acidity or alkalinity of a solution, ranging from acidic (low pH) to alkaline (high pH). Buffers are remarkable chemical solutions that possess the extraordinary ability to resist changes in pH when acids or bases are added to them.
Imagine a bustling city where the traffic flow is constantly fluctuating. Buffers act like the traffic controllers, maintaining the flow even as cars (acids or bases) enter or leave. They do this by containing a combination of a weak acid and its conjugate base.
The dissociation constant (pKa) of the weak acid determines its strength, and it represents the tendency of the acid to release protons (H+). The pKa value is closely related to the pH of the solution.
Weak acids and their conjugate bases work together to maintain a relatively constant pH within a certain buffer range. When you add an acid to a buffer, the weak acid neutralizes the excess protons, while the conjugate base helps to replenish the depleted protons. Conversely, when a base is added, the conjugate base neutralizes the excess hydroxide ions (OH-), while the weak acid helps to restore the lost protons.
The buffer capacity of a solution measures its ability to resist pH changes. It is directly proportional to the concentrations of the weak acid and its conjugate base. A higher buffer capacity means that the solution can withstand larger additions of acids or bases without experiencing significant pH changes.
The pH range of a buffer is determined by the strength of the weak acid and the ratio of its concentration to that of the conjugate base. Buffers with a wider pH range can maintain a relatively constant pH over a larger range of added acids or bases.
In summary, buffers play a vital role in maintaining pH stability, which is crucial for a wide range of chemical and biological processes. By understanding the principles of buffers, scientists and researchers can effectively control the pH of their systems, ensuring optimal conditions for various applications.
Understanding Buffers: What Makes a Good Buffer?
Weak Acids and Their Conjugate Bases
When we talk about acids, we measure their strength using a numerical value called pH. The pH scale runs from 0 to 14, with lower numbers indicating more acidity. Now, dissociation is a fancy word for when an acid breaks apart into positive and negatively charged ions. The more an acid dissociates, the stronger the acid. Acids that don’t dissociate much are known as weak acids.
Resistance to pH Change (Buffering Capacity)
Buffers are like the sturdy guardians of pH stability. They can withstand changes in pH, keeping it within a narrow range. This ability is known as buffering capacity.
Concentration of Buffer Components
Buffer capacity is directly proportional to the concentrations of the weak acid and its conjugate base. Conjugate bases are formed when weak acids donate a hydrogen ion (H+). The relative concentrations of these components determine the buffer’s pH.
Buffer Capacity
This is where it gets a bit technical. Buffer capacity is related to the concentrations of the buffer components and the pH range. The higher the buffer capacity, the more resistant to pH changes the buffer will be.
pH Range
pKa is a number that reveals the strength of an acid. It’s the negative logarithm of the dissociation constant, which tells us how much the acid dissociates. Weak acids have higher pKa values, meaning they dissociate less. The pH range of a buffer is determined by the weak acid’s strength and the ratio of its concentration to its conjugate base. The wider the pH range, the buffer can maintain relatively constant pH over a larger range of added acids or bases.
Understanding Buffers: What Makes a Good Buffer?
In the realm of chemistry, buffers play a crucial role in maintaining the delicate balance of pH, the measure of acidity or alkalinity. Buffers are solutions that resist changes in pH when small amounts of acid or base are added. Understanding what makes a good buffer is essential for a wide range of applications, from laboratory experiments to the functioning of biological systems.
One key aspect of buffers is the presence of a weak acid and its conjugate base. A weak acid is a molecule that partially dissociates into ions in water, releasing hydrogen ions (H+). Its conjugate base is the molecule that remains after the acid dissociates. The dissociation constant (pKa) of an acid is a measure of its strength, indicating the tendency of the acid to dissociate into ions. A lower pKa value indicates a stronger acid.
The buffer capacity of a buffer is a measure of its ability to resist changes in pH. A buffer with a high buffer capacity can withstand larger additions of acid or base without significant pH changes. Buffer capacity is determined by the concentrations of the weak acid and its conjugate base. The higher the concentrations, the greater the buffer capacity.
Buffer range refers to the pH range within which a buffer can maintain a relatively constant pH. The buffer range is determined by the strength of the weak acid and the ratio of its concentration to the concentration of its conjugate base. A wider buffer range allows the buffer to maintain a stable pH over a larger range of added acids or bases.
In summary, a good buffer is characterized by a weak acid with a low pKa (strong acid), a high concentration of both the weak acid and its conjugate base, and a wide buffer range. These factors ensure that the buffer can effectively neutralize small changes in pH, maintaining a stable chemical environment for various applications.
Understanding Buffers: What Makes a Good Buffer?
Resistance to pH Change (Buffering Capacity)
In the chemical world, pH is king. It measures the acidity or alkalinity of a solution, from 0 to 14. Buffers are the guardians of pH, protecting it from dramatic swings when acids or bases are lurking nearby.
Buffering Capacity: The Shield Against pH Changes
A buffer’s capacity, like a fearless knight’s, lies in its ability to resist pH changes. It’s like a force field that shields the solution from external invaders. The higher the buffering capacity, the more stalwart the defense against pH fluctuations.
The Magic of Weak Acids and Conjugate Bases
The key to a buffer’s resilience lies in the delicate dance between weak acids and their conjugate bases. These pairs are like yin and yang, always seeking balance. Weak acids, with their shy nature, only partially release their hydrogen ions (H+), while their conjugate bases, like valiant ions, eagerly accept them.
pH Range: The Buffer’s Domain
Each buffer has a unique pH range, a realm where it reigns supreme, maintaining a steady pH despite the addition of acids or bases. This range is determined by the strength of the weak acid and the ratio of its concentration to its conjugate base. The wider the range, the more versatile the buffer.
Understanding Buffers: What Makes a Good Buffer?
Buffers are essential components in maintaining a stable pH environment in biological systems and various chemical processes. They play a crucial role in resisting changes in pH when acids or bases are added, ensuring a stable pH within a narrow range. In this blog, we will dive into the concept of buffers, exploring their properties and the factors that make a good buffer.
Weak Acids and Their Conjugate Bases
The foundation of buffers lies in weak acids and their conjugate bases. The pH of a solution is a measure of its acidity or alkalinity, ranging from 0 to 14. Acidity refers to the presence of hydrogen ions (H+), while alkalinity signifies the presence of hydroxide ions (OH-). For weak acids, only a small fraction dissociates into ions, leaving a significant concentration of the undissociated acid. The pKa value, which is the negative log of the acid dissociation constant, indicates the strength of the acid. A lower pKa indicates a stronger acid.
Buffer Capacity
The ability of a buffer to resist changes in pH is known as its buffer capacity. When acids or bases are added to a buffer, it neutralizes them by donating or accepting protons. The buffer capacity is proportional to the concentrations of the weak acid and its conjugate base. A higher concentration of these components results in a higher buffer capacity.
pH Range
The pH range of a buffer is the interval within which it can maintain a relatively constant pH. It is determined by the strength of the weak acid and the ratio of its concentration to that of its conjugate base. Buffers with a wider pH range can maintain a stable pH over a larger range of added acids or bases.
Additional Factors
Beyond weak acids and conjugate bases, several other factors can impact buffer capacity, including:
- Temperature: Increased temperature generally decreases buffer capacity.
- Ionic strength: The presence of other ions in the solution can affect the ionization of the weak acid and its conjugate base.
- Complexation: The formation of complexes between buffer components and other ions can alter buffer capacity.
Buffers are essential for regulating pH in biological systems and chemical processes. Understanding the factors that contribute to a good buffer, such as weak acids, conjugate bases, buffer capacity, and pH range, is crucial for selecting and optimizing buffers for specific applications. By carefully considering these parameters, researchers and practitioners can leverage the power of buffers to maintain the desired pH environment in their experiments or processes.
Understanding Buffers: What Makes a Good Buffer?
Weak Acids and Their Conjugate Bases
The key to understanding buffers lies in the concept of weak acids and their conjugate bases. Weak acids, like acetic acid in vinegar, only partially dissociate in water, releasing hydrogen ions (H+) and their conjugate bases (acetate ions in the case of acetic acid).
Resistance to pH Change (Buffering Capacity)
Buffers have a remarkable ability to resist changes in pH. When a small amount of acid or base is added to a buffer, it doesn’t cause a significant shift in pH. This buffering capacity depends on two factors:
-
Buffer Range: The pH range where the buffer effectively resists pH changes.
-
Buffer Capacity: The ability of a buffer to neutralize added acids or bases without a significant pH change.
Concentration of Buffer Components
The buffering capacity of a buffer is directly proportional to the concentrations of both the weak acid and its conjugate base. This means that buffers with higher concentrations of these components will be more effective at resisting pH changes.
pH Range
The pH range of a buffer is determined by the strength of the weak acid. A stronger acid will have a narrower pH range, while a weaker acid will have a wider pH range. The wider the pH range, the more versatile the buffer will be.
Buffer Capacity: Proportional to Concentrations of Weak Acid and Conjugate Base
The buffering capacity of a buffer is directly proportional to the concentrations of the weak acid and its conjugate base. This means that buffers with higher concentrations of these components will be able to neutralize more acid or base without significantly changing the pH.
Understanding buffers is essential for various applications in chemistry and biology. By manipulating the concentration of weak acids and their conjugate bases, scientists can create buffers that maintain a desired pH within a specific range. This knowledge allows for precise control of pH in chemical reactions and biological systems, ensuring optimal conditions for optimal functioning.
Understanding Buffers: What Makes a Good Buffer?
Imagine you have a delicate chemical solution that requires a stable and balanced pH. This is where buffers come into play. Buffers are like chemical guardians, protecting your solution from pH fluctuations that can disrupt its integrity.
pH: The Measure of Acidity and Alkalinity
Every solution has a pH value, a number between 0 and 14 that indicates its acidity or alkalinity. A pH of 7 is considered neutral, while values below 7 are acidic and values above 7 are basic.
Weak Acids and Their Conjugate Bases: The Secret to Buffering
Buffets are made up of weak acids and their conjugate bases. Weak acids partially dissociate in water, releasing hydrogen ions (H+) and leaving behind charged molecules called anions. The conjugate base is the anion that forms when a weak acid donates a hydrogen ion.
Buffering Capacity: The Resistance to pH Change
The buffering capacity of a buffer is its ability to resist changes in pH. It’s determined by the concentrations of the weak acid and its conjugate base in the solution. When acids or bases are added to a buffered solution, the buffer components react to neutralize their effects, keeping the pH relatively constant.
Concentration of Buffer Components: The Key to Success
The concentration of the weak acid and conjugate base plays a crucial role in buffering capacity. Higher concentrations of these components provide greater resistance to pH change. The relative concentrations of these components also influence the pH of the buffer.
Buffer Capacity: The Measure of a Buffer’s Strength
The buffer capacity of a buffer is determined by both the concentrations of its components and the pH range over which it can maintain a constant pH. Buffers with a higher buffer capacity can resist larger changes in pH.
pH Range: The Right Buffer for the Job
The pH range of a buffer is determined by the strength of the weak acid and the ratio of its concentration to the conjugate base. Buffers with a wider pH range can maintain a relatively constant pH over a larger range of added acids or bases.
By understanding these key concepts, you can choose the right buffer for your application, ensuring the stability and integrity of your delicate chemical solutions.
Understanding Buffers: What Makes a Good Buffer?
In the world of chemistry, buffers play a crucial role in maintaining stable pH levels in various solutions. But what exactly are buffers and what makes them so effective?
Weak Acids and Their Conjugate Bases
Imagine a weak acid like acetic acid (vinegar). When it dissolves in water, it partially dissociates, releasing hydrogen ions (H+) and its conjugate base, acetate ions (CH3COO-). The strength of an acid is measured by its pKa value, which indicates the tendency to dissociate.
Resistance to pH Change (Buffering Capacity)
The magic of buffers lies in their ability to resist pH changes. When small amounts of acid or base are added to a buffer solution, the buffer neutralizes these additions, keeping the pH relatively constant. This is the essence of buffering capacity, which depends on the concentrations of the weak acid and its conjugate base.
Concentration of Buffer Components
The higher the concentrations of both the weak acid and its conjugate base, the stronger the buffer will be. This is because a higher concentration of the weak acid provides more H+ ions to neutralize added bases, while the higher concentration of the conjugate base provides more OH- ions to neutralize added acids.
pH Range
The pH range of a buffer is also a critical factor. It is determined by the strength of the weak acid and the ratio of its concentration to that of the conjugate base. Buffers with wider pH ranges can maintain stable pH levels over a broader range of added acids or bases.
Buffer Capacity
The overall effectiveness of a buffer is determined by its buffer capacity, which is directly influenced by both the buffer component concentrations and the pH range. A buffer with a higher buffer capacity can resist greater pH changes without significant variation.
In summary, understanding buffers requires grasping the concepts of weak acids, conjugate bases, buffer capacity, and pH range. By skillfully manipulating these factors, chemists can create buffers tailored to specific applications, ensuring stable pH conditions in various chemical and biological systems.
Understanding Buffers: What Makes a Good Buffer?
In the realm of chemistry, buffers play a crucial role in maintaining the delicate pH balance of solutions. A buffer is essentially a chemical solution that resists changes in pH when small amounts of acid or base are added. This ability, known as buffer capacity, is what distinguishes a good buffer from a mediocre one.
One of the key factors that determines buffer capacity is the concentration of the buffer components. A buffer typically consists of a weak acid and its conjugate base. The dissociation constant of the weak acid (a measure of its strength) and the relative concentrations of the acid and base influence the pH of the buffer solution.
The higher the buffer capacity, the more effectively the buffer can resist pH changes. This is because a buffer with higher capacity has a larger pool of buffer components to neutralize added acids or bases. Consequently, the pH of a solution with a high buffer capacity will remain relatively constant even when subjected to significant pH perturbations.
The pH range of a buffer is also an important consideration. A buffer’s pH range is the range of pH values over which it can maintain a relatively constant pH. The pH range is determined by the pKa value of the weak acid and the ratio of its concentration to the concentration of its conjugate base.
In general, a wider pH range indicates a more versatile buffer. A buffer with a wide pH range can maintain a relatively constant pH over a larger range of added acids or bases, making it suitable for a variety of applications.
Therefore, when selecting a buffer for a particular application, it is essential to consider the desired pH range and the required buffer capacity. A higher buffer capacity will resist larger pH changes and maintain a more stable pH under varying conditions.
Understanding Buffers: What Makes a Good Buffer?
In the realm of chemistry, understanding buffers is crucial for maintaining equilibrium in delicate solutions. Buffers are the unsung heroes that keep pH stable, preventing harmful fluctuations that can disrupt biochemical processes.
At the heart of every buffer lies a weak acid and its conjugate base. Weak acids, as the name suggests, are reluctant to give up their protons (H+ ions). When they do, they form their conjugate bases, which are ready and willing to accept protons. This delicate dance between the weak acid and its conjugate base is what gives buffers their remarkable ability to resist pH changes.
The buffer capacity, a measure of a buffer’s strength, depends on the concentrations of the weak acid and its conjugate base. As these concentrations increase, so does the buffer’s capacity to neutralize incoming acids or bases, maintaining a constant pH.
The pH range of a buffer is determined by the strength of the weak acid and the relative concentrations of the acid and its conjugate base. A wider pH range allows the buffer to handle a larger range of added acids or bases without significant pH fluctuations.
In essence, a good buffer is characterized by a strong weak acid (one that doesn’t readily dissociate) and a high concentration of both the weak acid and its conjugate base. This combination ensures that the buffer has a high buffer capacity and a wide pH range, enabling it to effectively counteract the effects of added acids or bases, maintaining a stable pH environment.
Understanding Buffers: Unlocking the Key to Stable pH Levels
In the intricate world of chemistry, understanding buffers is crucial for maintaining equilibrium and stability in countless chemical reactions. A buffer is a solution that resists changes in pH (acidity or alkalinity), safeguarding crucial processes that rely on a specific pH range.
Weak Acids and Their Conjugate Bases
Buffers utilize the power of weak acids and their conjugate bases. When a weak acid dissolves in water, it releases protons (H⁺ ions), resulting in a mildly acidic solution. The conjugate base, formed when the acid loses a proton, acts as a reservoir of hydrogen ions, ready to neutralize any added acids.
Buffering Capacity: The Resistance to pH Change
The buffering capacity measures a buffer’s ability to maintain a constant pH. This capacity depends on the concentrations of both the weak acid and its conjugate base. When added acids or bases disrupt the pH balance, the buffer neutralizes them by releasing or absorbing protons, effectively resisting the pH change.
Concentration of Buffer Components
The concentrations of the buffer components play a significant role in determining its buffering capacity. Higher concentrations of both the weak acid and its conjugate base result in a stronger buffer, more resistant to pH changes. Conversely, lower concentrations weaken the buffer’s capacity.
Buffer Range: Maintaining pH Equilibrium
The buffer range defines the pH range over which the buffer can effectively maintain a relatively constant pH. Buffers with a wider pH range can handle more substantial additions of acids or bases without significant pH fluctuations. This range is influenced by the strength of the weak acid and the ratio of its concentration to its conjugate base. Stronger acids result in narrower pH ranges.
Unlocking Buffer Potential
Buffers find diverse applications in various fields, including:
- Biology: Regulating pH levels in cells and body fluids
- Chemistry: Stabilizing reactions and maintaining optimal conditions for experiments
- Industry: Controlling acidity in manufacturing processes and wastewater treatment
Understanding the principles of buffers empowers us to harness their ability to maintain stable pH levels, ensuring optimal conditions for biological processes, chemical reactions, and industrial applications.