Understanding Bromine: Valence Electrons, Properties, And Reactivity
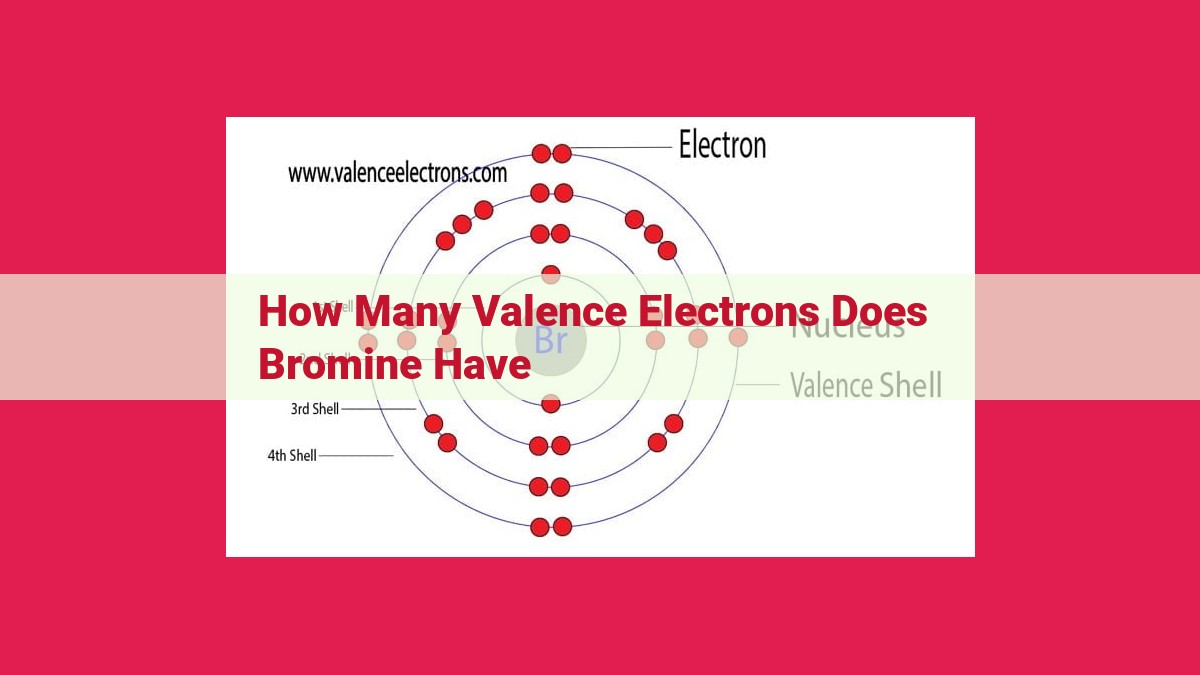
Bromine, a halogen element with an atomic number of 35, possesses seven valence electrons. These valence electrons, located in its outermost energy level, play a crucial role in determining its chemical properties and bonding behavior. Bromine’s tendency to form covalent bonds with other elements is attributed to its need to stabilize its valence electron configuration by gaining or sharing electrons. This shared characteristic of seven valence electrons among halogens, including bromine, explains their similar reactivity and behavior in chemical reactions.
Valence Electrons: Unleashing the Power of Chemical Interactions
In the realm of chemistry, understanding the behavior of atoms is paramount. One key player in this dynamic world is the valence electron, the outermost electron in an atom’s orbit. Valence electrons hold the key to unraveling the chemical properties and bonding capabilities of elements.
Valence electrons are like the social butterflies of the atomic world, eager to interact and form bonds with their neighbors. The number of valence electrons an element possesses significantly influences its chemical personality.
Take bromine, a fascinating element from Group 17 of the periodic table. With an atomic number of 35, bromine’s electron configuration reveals a hidden secret: it boasts seven valence electrons. These seven electrons, like mischievous imps, dance around the atom’s nucleus, longing to connect with other elements and shape the chemical world.
Bromine’s valence electrons play a pivotal role in its chemical reactivity. Like a magnet, these electrons attract other elements, forming strong bonds and forging new molecules. This versatility makes bromine an indispensable player in a wide range of chemical reactions, from disinfecting agents to fire retardants.
Intriguingly, bromine is not alone in its septet of valence electrons. All elements in Group 17, known as halogens, share this common trait. Chlorine, fluorine, iodine, and astatine all possess seven valence electrons, giving them similar chemical properties. This shared electron configuration enables halogens to participate in a multitude of reactions, making them essential to the tapestry of chemical life.
Meet Bromine, the Enigmatic Halogen
Prepare yourself to embark on a thrilling scientific adventure as we delve into the captivating world of chemistry. Our journey begins with a mysterious element that lurks in Group 17 of the periodic table, the enigmatic bromine.
Bromine, a nonmetal, proudly occupies the 35th spot in the pecking order of elements, its atomic number a testament to its unique identity. Its home lies within the halogen family, a group of elements renowned for their exceptional reactivity and affinity for forming bonds.
Bromine stands apart from its peers with its distinctive reddish-brown hue and liquid state at room temperature, making it an intriguing subject of study. It’s a volatile substance, readily evaporating into a pungent, reddish-brown gas. This peculiar element holds a profound significance in various industries, including the production of flame retardants, dyes, and disinfectants.
Counting Bromine’s Valence Electrons: Uncovering the Secrets of its Chemical Behavior
Understanding Electron Configuration: The Key to Valence Electrons
Every atom, like a tiny world, is composed of a nucleus surrounded by a cloud of electrons arranged in different energy levels or shells. The outermost shell, known as the valence shell, holds a special group of electrons known as valence electrons. These electrons play a pivotal role in determining an element’s chemical properties and its ability to form bonds with other elements.
Bromine’s Atomic Identity: Element 35
Bromine is a fascinating element that belongs to Group 17 of the periodic table, commonly known as the halogens. It occupies the 35th position, indicating that its atoms have 35 electrons.
Diving into Bromine’s Electron Configuration
To determine bromine’s valence electrons, we need to examine its electron configuration. This arrangement of electrons in energy levels reveals the number of electrons in each shell. For bromine, its electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
Unveiling the Seven Valence Electrons
The key to finding valence electrons is focusing on the outermost energy level, which is the 4th energy level for bromine. In this level, we count seven electrons: two in the 4s subshell and five in the 4p subshell. These seven electrons are bromine’s valence electrons.
The Significance of Valence Electrons: Bromine’s Chemical Behavior
Bromine’s seven valence electrons play a crucial role in its chemical reactivity. With seven valence electrons, bromine has a strong tendency to gain one electron to complete its valence shell and achieve a stable electron configuration like a noble gas. This inherent desire to complete its valence shell drives bromine’s high reactivity and its ability to form bonds with other elements, especially those with low ionization energies like alkali metals.
The Significance of Valence Electrons: Unlocking Bromine’s Chemical Secrets
In the world of chemistry, understanding valence electrons is key to unraveling the behavior of elements. Valence electrons are the electrons in an atom’s outermost energy level, and they play a pivotal role in chemical reactions. Let’s delve into the intriguing case of bromine to illustrate this concept.
Bromine, a member of the halogen group, is a highly reactive element with a distinctive reddish-brown hue. Its unique chemical properties, such as its tendency to form bonds with other elements, can be attributed to its seven valence electrons.
These valence electrons dance around the bromine nucleus, eager to interact with other atoms. They determine bromine’s chemical bonding behavior, influencing its ability to form both covalent and ionic bonds. Covalent bonding occurs when bromine shares its valence electrons with another atom, creating a strong chemical bond. Ionic bonding, on the other hand, involves the transfer of valence electrons from bromine to another atom, resulting in the formation of positively and negatively charged ions.
The presence of seven valence electrons grants bromine a high electronegativity, a measure of its ability to attract electrons from other atoms. This electronegativity allows bromine to readily form bonds with elements that have lower electronegativities, such as metals.
Furthermore, bromine’s valence electrons enable it to participate in redox reactions, which involve the transfer of electrons between atoms or molecules. In these reactions, bromine can act as either an oxidizing agent (accepting electrons) or a reducing agent (donating electrons), depending on the specific reaction conditions.
In conclusion, the seven valence electrons of bromine shape its chemical reactivity and bonding behavior. By understanding the significance of valence electrons, we can gain a deeper insight into the fascinating world of chemistry and the unique properties of elements like bromine.
Halogens: A Shared Trait of Seven Valence Electrons
- Overview of Group 17 elements (halogens) and their shared chemical properties
- Explanation of how their common number of valence electrons contributes to their reactivity
Halogens: United by Seven Valence Electrons
In the world of chemistry, the periodic table offers a roadmap to understanding the elements and their diverse properties. Among these elements, a fascinating group emerges: the halogens, residing under Group 17. What truly sets them apart is their shared secret—they all possess seven valence electrons.
Valence electrons, those outermost electrons in an atom’s energy level, play a crucial role in determining an element’s reactivity and bonding behavior. For the halogens, their seven valence electrons grant them an insatiable desire to complete their outermost electron shell by forming bonds with other elements.
This shared trait of seven valence electrons unites the halogens in a symphony of chemical similarities. They all exist as diatomic molecules, forming strong bonds between two identical atoms, such as in the case of chlorine (Cl2) and bromine (Br2). Their high electronegativity, a measure of their ability to attract electrons, makes them relentless in forming ionic bonds with metals, resulting in compounds like sodium chloride (NaCl) and potassium bromide (KBr).
Moreover, these seven valence electrons empower the halogens to participate in covalent bonding, sharing electrons with other non-metals to create stable molecules. From the pungent chlorine gas used in water purification to the essential iodine in thyroid hormone, the halogens leave an undeniable mark on our world.
In the grand scheme of chemistry, the halogens stand out as a cohesive family, bound together by their shared characteristic—seven valence electrons. This unifying trait shapes their reactivity, bonding behavior, and ultimately, their profound impact on the chemical processes that shape our lives.