Understanding Barium’s Neutron Count And Its Significance In Physics And Industry
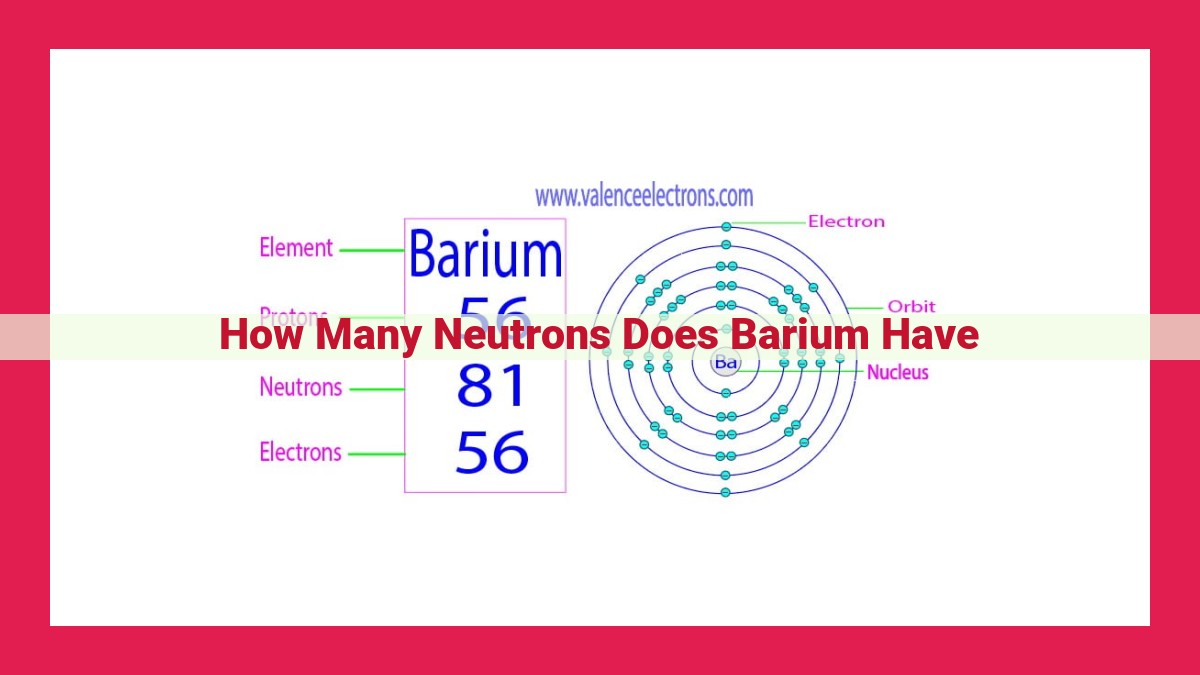
Barium, an element in the periodic table, has 81 protons and 56 electrons. Determining the number of neutrons requires calculating the difference between the mass number and the atomic number: 138 (mass number) – 81 (atomic number) = 57 neutrons. These neutrons stabilize the atomic nucleus, contributing to barium’s physical and chemical properties, such as its malleability and reactivity. Neutron composition plays a crucial role in the stability and behavior of barium, affecting its applications in alloys, pigments, and scientific research. Understanding the number of neutrons in barium is essential for comprehending its properties and industrial significance.
- Introduce barium as a chemical element and its significance in the periodic table.
In the realm of elements, barium stands as a captivating subject, alluring scientists and enthusiasts alike. As the 56th element on the periodic table, barium holds a pivotal position, embodying the very essence of chemistry’s organizing principles. Its atomic properties and the intricate dance of particles within its nucleus have profound implications for its unique characteristics and diverse applications.
Journey with us as we delve into the fascinating world of barium, unraveling its atomic structure, calculating the number of neutrons that grace its nucleus, and exploring its remarkable properties. We’ll uncover the crucial role neutrons play in shaping barium’s stability and behavior, and we’ll shed light on the practical uses that have made barium an indispensable element in our technological and industrial landscape.
Delving into the Atomic Structure of Barium: A Journey into the Heart of Matter
At the core of every element lies an intriguing world of particles, protons, and electrons, forming the very foundation of its existence. In this captivating exploration, we embark on a journey to unravel the atomic structure of Barium (Ba), an element with unique properties that have shaped its role in our world.
The Building Blocks of Barium
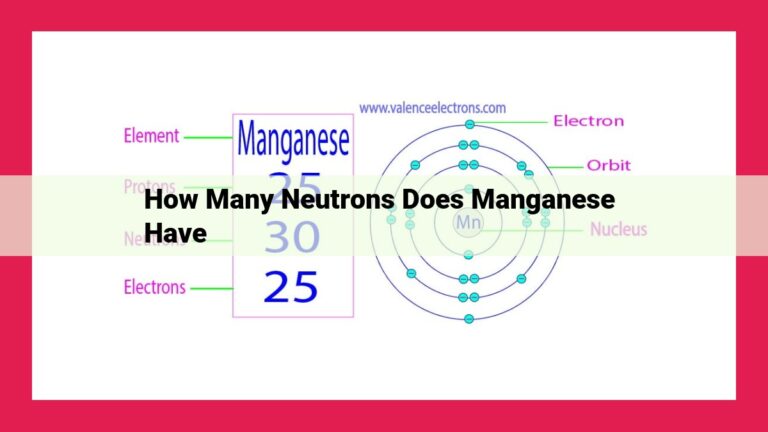
The atom of barium, like all other elements, consists of a tiny nucleus at its center, which houses positively charged protons and neutral neutrons. Orbiting this nucleus like a miniature solar system are negatively charged electrons. The number of protons in an atom defines its atomic number, which for barium is 56. This characteristic number identifies barium as a unique element on the periodic table.
Unveiling the Neutron Count
The number of neutrons in an atom plays a crucial role in its stability and behavior. To calculate the number of neutrons in barium, we utilize a simple formula:
Mass number (A) – Atomic number (Z) = Number of neutrons (N)
Given that the mass number of barium is 137, the number of protons (atomic number) is 56, we can determine the number of neutrons:
137 - 56 = 81
Therefore, barium atoms contain 81 neutrons.
The Significance of Neutrons
Neutrons, though electrically neutral, hold immense significance in the atomic structure of barium. They act as a stabilizing force within the nucleus, counteracting the repulsive forces between positively charged protons. This ensures the stability of the barium atom, preventing it from disintegrating.
Furthermore, the composition of neutrons has a direct impact on the isotopes of barium. Isotopes are atoms of the same element with varying numbers of neutrons. Barium has several stable isotopes, each with a slightly different neutron count. This variation affects the atomic mass and certain physical properties of the isotopes.
Calculating the Enigmatic Number of Neutrons in Barium
Embarking on a journey into the atomic realm, we encounter barium, an alluring element that captivates us with its significance within the tapestry of the periodic table. To delve into the enigma that is barium, we must unravel the secrets of its atomic structure, including the enigmatic number of neutrons that reside within its nucleus.
To comprehend this concept, we must grasp the essence of atomic structure. This intricate dance of protons and electrons plays a pivotal role in defining an element’s identity. Each element possesses a unique atomic number, which represents the number of protons within its nucleus. This number, an immutable fingerprint, distinguishes one element from another.
Alongside protons, the nucleus also harbors neutrons, uncharged particles that contribute to the element’s mass. The interplay between protons, electrons, and neutrons determines an element’s stability, behavior, and properties.
In the case of barium, its atomic number is 56. This indicates that each barium atom contains 56 protons. To determine the number of neutrons, we turn to a fundamental relationship that governs the atomic realm:
Mass Number = Atomic Number + Number of Neutrons
The mass number represents the total number of protons and neutrons within the nucleus. Barium’s mass number is 138.
Using this equation, we can solve for the number of neutrons:
Number of Neutrons = Mass Number – Atomic Number
Plugging in the values for barium, we find:
Number of Neutrons = 138 – 56 = 82
Thus, each barium atom contains 82 neutrons. These neutrons play a crucial role in stabilizing the barium nucleus and shaping its unique properties. They are the silent partners, the unsung heroes that contribute to the very essence of this fascinating element.
Properties of Barium: A Versatile Element with Distinct Characteristics
Physical Properties:
Barium is a fascinating element that belongs to the alkaline earth family. It’s a soft, silvery-white metal that’s not quite as soft as sodium or potassium but malleable enough to be cut with a knife. Its high reactivity makes it unlikely to be found in its pure form in nature, but it’s commonly found combined with other elements in minerals.
Chemical Properties:
Chemically, barium is a reactive metal that readily loses its two valence electrons to form positive ions. This makes it an excellent reducing agent, meaning it can give up electrons to other elements. Barium also reacts vigorously with water, producing barium hydroxide and liberating hydrogen gas.
Natural Occurrence:
Barium is relatively abundant in the Earth’s crust, ranking 14th among all elements. It’s primarily found in the mineral barite, which is barium sulfate. Barite is commonly used as a weighting agent in drilling fluids and as a filler in paints and plastics.
Other Properties:
In addition to its physical and chemical properties, barium also exhibits some unique characteristics. It has a high melting point (850°C) and a boiling point (2,170°C), making it useful for high-temperature applications. It’s also a good conductor of electricity, though not as good as metals like copper or silver.
The Role of Neutrons in Barium: A Nuclear Balancing Act
Neutrons, the unsung heroes of the atomic nucleus, play a crucial role in the stability and behavior of barium. These subatomic particles, devoid of charge, serve as the glue that holds the positively charged protons together, maintaining the integrity of the nucleus.
Barium, a soft, silvery-white metal, owes its stability to the perfect balance between protons and neutrons within its atomic nucleus. With 56 protons, barium possesses 81 neutrons, resulting in a mass number of 137. This neutron-rich composition contributes to the element’s inherent stability, preventing it from undergoing radioactive decay.
Without a sufficient number of neutrons, the protons would repel each other, causing the nucleus to shatter. Too many neutrons, on the other hand, would weaken the nucleus’s structure, making it susceptible to decay. Barium’s optimal neutron composition ensures its longevity and allows it to retain its unique properties.
Furthermore, the neutron composition of barium influences its chemical reactivity. The greater the neutron-to-proton ratio, the less reactive the element. Barium’s high neutron-to-proton ratio contributes to its low reactivity, making it resistant to corrosion and oxidation.
In conclusion, neutrons play a vital role in the stability and behavior of barium. Their presence in the atomic nucleus prevents radioactive decay, enhances chemical stability, and maintains the element’s unique properties. Without these unsung heroes, barium would be a much different element, its existence and applications forever altered.
Applications of Barium
- Mention industrial and scientific applications where barium is used, such as in alloys, pigments, and pyrotechnics.
Applications of Barium: From Alloys to Pyrotechnics
Industrial Applications
In the realm of industry, barium finds its place in the form of alloys. These alloys exhibit remarkable properties, making them ideal for specific applications. For instance, barium-based alloys are employed in the production of automotive parts, where their exceptional strength and hardness are highly valued.
Scientific Applications
Beyond its industrial uses, barium also plays a crucial role in scientific endeavors. It is a key component of fluorescent tubes, where it contributes to the emission of visible light. Moreover, its high atomic number makes barium an essential element in X-ray imaging applications.
Artistic Expressions
In the world of art, barium unveils its creative side. It is an indispensable ingredient in pigments used to create brilliant whites and other vivid colors. These pigments find their way into paints, ceramics, and even cosmetics, adding a touch of enchantment to our visual experiences.
Pyrotechnic Displays
When it comes to pyrotechnics, barium takes center stage. It is the heart of fireworks, responsible for those captivating green and blue hues that adorn the night sky. These vibrant colors result from barium’s unique emission spectrum, making it an indispensable element for creating awe-inspiring displays.