Understanding Amylase: The Enzyme That Breaks Down Starch And Its Versatile Applications
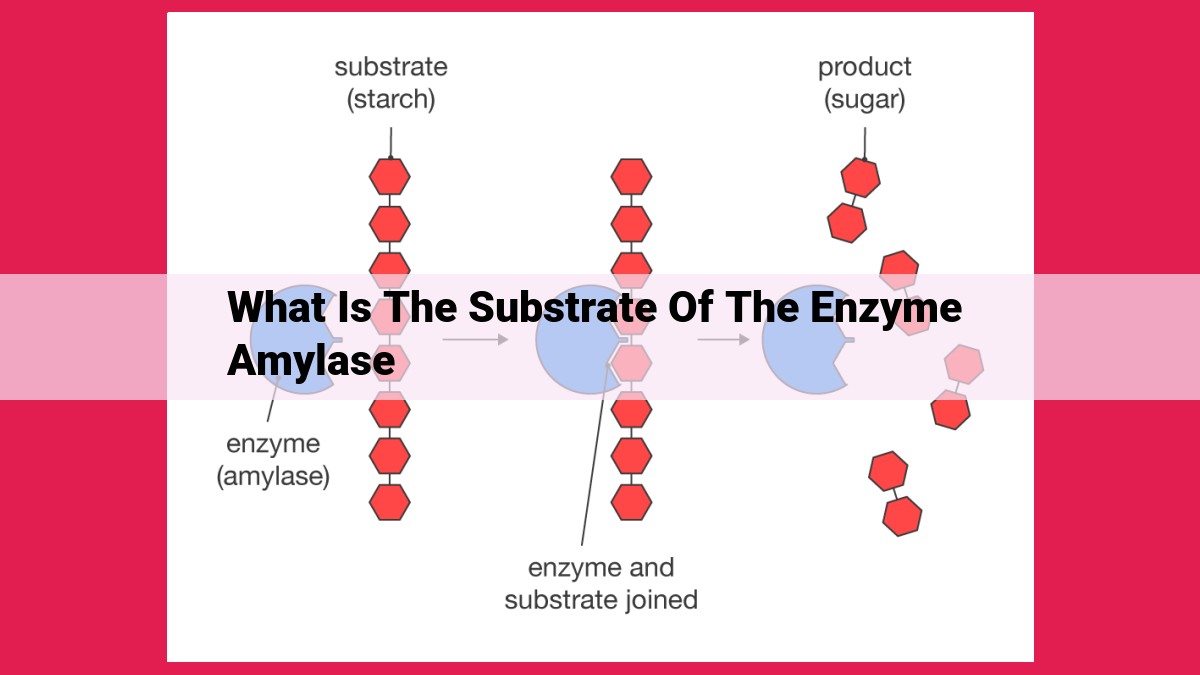
Amylase, a crucial enzyme, primarily targets starch, a complex carbohydrate composed of glucose units linked in alpha-1,4 and alpha-1,6 glycosidic bonds. Starch serves as the primary energy reserve in plants, providing sustenance and structural support. Other substrates include glycogen, the animal equivalent of starch, dextrin, an intermediate product of starch breakdown, and maltose, a disaccharide produced by amylase. Soluble starch, a modified form, possesses enhanced properties, while pullulan, a microbial polysaccharide, presents starch-like characteristics.
Starch: The Essential Fuel for Life’s Processes
In the realm of carbohydrates, starch reigns supreme as the primary substrate for amylase, an enzyme that orchestrates the breakdown of complex carbohydrates into simpler sugars. Let’s embark on a journey to unravel the intricacies of starch, exploring its structure, composition, and fundamental role as the lifeblood of cellular processes.
Unveiling the Structure of Starch: A Complex and Remarkable Architecture
Starch is a polymeric carbohydrate composed of numerous glucose units linked together by glycosidic bonds. These glucose units can be arranged in two distinct forms: amylose, a linear chain of glucose molecules, and amylopectin, a highly branched structure resembling a tree’s canopy. This intricate architecture provides starch with unique properties, making it an ideal energy reserve for both plants and animals.
Composition and Importance: Starch as the Cornerstone of Life
Starch is composed primarily of glucose, a simple sugar that serves as the body’s primary energy source. The complex structure of starch allows for its gradual breakdown into glucose, providing a sustained release of energy over time. This sustained energy release is critical for a myriad of cellular processes, including growth, metabolism, and cellular respiration.
Beyond Glucose: The Diverse Applications of Starch
While its role as an energy source is paramount, starch also finds applications in various industries. In the food industry, it thickens sauces, stabilizes emulsions, and contributes to the texture of processed foods. In the pharmaceutical industry, starch is employed as a binder and disintegrant in tablets and capsules. Its versatility extends to the paper and textile industries, where it enhances product quality and durability.
Starch stands as a cornerstone of life, providing essential energy for cellular processes and supporting diverse industries. Its intricate structure and diverse applications showcase the remarkable versatility of nature’s chemistry. As we continue to unravel the secrets of this remarkable carbohydrate, we gain a deeper appreciation for its vital role in life’s symphony.
Glycogen: The Energy Fuel of Animals
In the realm of energy storage, starch reigns supreme in plants, but its animal counterpart, glycogen, is equally indispensable. Like starch, glycogen is a complex carbohydrate that serves as a primary energy reserve in animals.
Structurally, glycogen and starch share similarities. Both are composed of glucose molecules, linked together in a highly branched network. However, glycogen is more branched than starch, resembling a dense, bushy structure. This increased branching allows for rapid breakdown and release of glucose, making glycogen an ideal source of readily available energy.
As the primary energy reserve in animals, glycogen is predominantly stored in the liver and muscles. When energy is required, glycogen is broken down by enzymes into glucose, which can then be metabolized for cellular respiration. In the liver, glycogen breakdown helps maintain glucose levels in the bloodstream, ensuring a steady supply of energy to the body. In muscles, glycogen acts as a direct fuel for muscular contractions.
Unlike starch, which is found in plant cells, glycogen is exclusive to animal cells. This distinction reflects the different energy needs of plants and animals. Plants have access to sunlight for photosynthesis, providing them with a continuous source of energy. In contrast, animals must rely on stored energy reserves, such as glycogen, to sustain their activity.
Glycogen’s importance as an energy store is undeniable, making it essential for the proper functioning of animals. Whether powering muscular movements or maintaining blood sugar levels, glycogen plays a crucial role in ensuring the energy availability necessary for life.
Dextrin: A Starch Hydrolysate
Dextrin, an unsung hero in the world of carbohydrates, plays a pivotal role in the breakdown of starch, the primary food reserve for both plants and animals. It’s a maltodextrin, an intermediate product formed when starch encounters enzymes like amylase, which break down complex carbohydrates into simpler sugars.
Imagine a journey through the fascinating world of dextrin. It starts with starch, a complex molecule composed of interconnected glucose units. When amylase does its magic, it breaks down these complex starch chains into smaller fragments, releasing dextrins. Dextrins, like stepping stones in a metabolic pathway, are intermediates in the conversion of starch to glucose, the body’s preferred energy source.
The properties of dextrin make it a versatile substance. It’s soluble, giving it the ability to dissolve in water, and has a low viscosity, making it easy to handle. Dextrins also possess a pleasant taste, adding a touch of sweetness to food products.
In the culinary realm, dextrin finds its niche as a thickening agent. It’s a key ingredient in many sauces, gravies, and dressings, where it adds body and smoothness. Its ability to form gels makes it useful in confectionery applications, creating chewy textures in candies and marshmallows.
Beyond the kitchen, dextrin finds diverse uses in industries such as textiles, papermaking, and adhesives. In textile manufacturing, it’s used as a sizing agent, providing strength and smoothness to fabrics. In papermaking, it acts as a binder, holding paper fibers together. And in adhesives, dextrin offers excellent bonding properties, making it a valuable component in various applications.
As you delve into the world of dextrin, remember its vital role in the breakdown of starch and its versatility in various industries. It’s a testament to the intricate workings of our natural world, where even intermediate products like dextrin play a significant part in the symphony of life.
Maltose: The Sweet Link in Starch Breakdown
In the realm of carbohydrates, maltose stands out as the sweet result of the enzymatic dance performed by amylase, the enzyme responsible for breaking down starchy molecules. As a disaccharide, maltose is composed of two glucose molecules joined by an alpha-1,4-glycosidic bond.
Structure and Sweetness
Maltose’s linear structure resembles that of a dumbbell, with two glucose subunits connected in the middle. This compact arrangement gives it a higher solubility than starch, making it more readily available for absorption. Maltose’s sweetness is approximately 60% that of sucrose, the common table sugar.
Biological Significance
Maltose plays a crucial role in the metabolism of carbohydrates. After amylase breaks down starch into maltose, it is further broken down into glucose by the enzyme maltase. Glucose, the body’s primary energy source, is then used to fuel cellular processes.
Metabolism of Maltose
The metabolism of maltose involves two steps:
- Maltase Breakdown: On the surface of the small intestine, maltase splits maltose into two glucose molecules.
- Glucose Absorption: The glucose molecules are then absorbed into the bloodstream and distributed throughout the body for energy production.
Applications of Maltose
Beyond its biological significance, maltose finds numerous applications in the food industry:
- Sweetener: In baking and confectionery, maltose is used as a natural sweetener to enhance flavor and texture.
- Flavor Enhancer: It is added to foods such as beer, soy sauce, and processed meats as a flavor enhancer.
- Maltodextrin Production: Maltose can undergo further enzymatic breakdown to produce maltodextrin, a polysaccharide used as a thickening agent and stabilizer in food products.
Maltose, the humble disaccharide, plays a vital role in the breakdown and utilization of starch in our bodies. Its distinct structure, sweet taste, and biological significance make it an indispensable player in the realm of carbohydrates. From providing energy to enhancing flavors, maltose contributes to a myriad of processes that sustain our daily lives.
Soluble Starch: A Modified Form for Enhanced Applications
As we delve into the intriguing world of carbohydrates, we encounter starch, the primary energy storage molecule found in plants. It serves as the foundation for various industrial and culinary applications. However, modifying starch’s properties can further expand its utility. Enter soluble starch, a remarkable form that has undergone a chemical transformation to unlock enhanced capabilities.
Chemical Modification Process
Soluble starch is produced by treating native starch with acids or enzymes. This process breaks down the complex structure of starch into smaller, more water-soluble molecules. The extent of modification can vary, resulting in a range of solubility levels.
Enhanced Properties
The modification process imparts several desirable properties to soluble starch:
-
Increased Solubility: Native starch is insoluble in cold water, limiting its applications. Soluble starch, on the other hand, easily dissolves in cold water, forming a clear, viscous solution. This enhanced solubility makes it ideal for use as a thickener or stabilizer in various food and industrial products.
-
Reduced Retrogradation: When native starch is heated and cooled, it tends to recrystallize, forming a hard, brittle gel known as retrogradation. Soluble starch exhibits reduced retrogradation, allowing products containing it to remain soft and pliable over time.
-
Improved Clarity: Native starch solutions can be cloudy due to the presence of starch granules. Soluble starch, with its smaller molecule size, provides clearer solutions, making it suitable for applications where transparency is essential.
Versatile Applications
The enhanced properties of soluble starch make it a versatile material with a wide range of applications:
-
Food Industry: As a stabilizer, thickener, and gelling agent, soluble starch finds use in sauces, soups, desserts, and baked goods. It imparts texture and stability while enhancing visual appeal.
-
Adhesives: The adhesive properties of soluble starch make it useful in the production of paper, cardboard, and textiles. It provides a strong bond that resists moisture and heat.
-
Pharmaceuticals: In the pharmaceutical industry, soluble starch serves as a binder and disintegrant in tablet formulations, ensuring proper drug delivery.
-
Industrial: Soluble starch is employed in the production of coatings, inks, and construction materials. Its versatility and cost-effectiveness make it a valuable ingredient in a variety of industrial applications.
Soluble starch is a remarkable modification of native starch, unlocking enhanced properties that make it suitable for a wide range of applications. Its increased solubility, reduced retrogradation, and improved clarity, coupled with its versatility and cost-effectiveness, position soluble starch as a valuable material in various industries. It exemplifies the power of modifying natural substances to cater to our evolving needs.
Pullulan: A Versatile Microbial Polysaccharide with Starch-Like Properties
In the realm of carbohydrates, pullulan stands out as an exceptional polysaccharide with remarkable properties that mirror those of starch. This microbial treasure, produced by the bacterium Aureobasidium pullulans, has captivated scientists and industrialists alike due to its diverse applications in various fields.
Origin and Structure
Pullulan is a water-soluble, linear homopolysaccharide composed of repeating maltotriose units. Unlike starch, which consists of a mixture of amylose and amylopectin, pullulan possesses a uniform structure. Its biogenesis involves the polymerization of maltotriose molecules by the enzyme pullulan synthase.
Properties and Applications
Pullulan’s unique structure endows it with an array of properties that make it suitable for a wide range of applications. It is a viscous and highly adhesive substance, making it ideal for food additives, thickeners, and stabilizers. In the pharmaceutical industry, pullulan is employed as a drug carrier and encapsulating agent.
Its exceptional film-forming ability has led to its use in biodegradable plastics, coatings, and membranes. Moreover, pullulan’s biocompatibility and low toxicity make it a promising candidate for biomedical applications such as tissue engineering and drug delivery.
Potential Uses
The versatility of pullulan extends beyond its current applications. Ongoing research suggests its potential in the development of functional foods, prebiotics, and biofuels. Its ability to form gels and films opens up possibilities in various sectors, including agriculture, cosmetics, and environmental remediation.
As scientists continue to delve into the captivating world of pullulan, its full potential is yet to be fully unleashed. With its unique properties and diverse applications, this microbial polysaccharide is poised to revolutionize industries and play a significant role in shaping the future of sustainable and innovative technologies.